Abstract
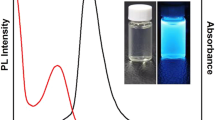
Fluorescent silicon (Si) nanoparticles (SiNPs) hold great promise for innumerable biological and biomedical applications owing to their unique optical properties and negligible toxicity. In this article, we present a new traditional Chinese medicine (TCM) molecule-assisted chemical synthetic strategy, suitable for the production of multifunctional small-sized (diameter: ~ 3.7 nm) SiNPs in a facile and rapid (~ 10 min) manner. Of particular significance, the resultant SiNPs simultaneously exhibited robust and stable fluorescence (photoluminescence quantum yield (PLQY): ~ 15%), as well as intrinsic anti-cancer efficacy with excellent selectivity toward cancer cells. Taking advantage of these unique merits, we further employed these novel fluorescent anti-cancer SiNPs (AC-SiNPs) for the fluorescence tracking and treatment of tumors, demonstrating long-term (~ 18 days) inhibition of tumor growth in tumor-bearing mice. Consequently, we believe this new TCM-assisted chemical synthetic method is highly attractive for designing silicon nanostructures featuring multiple functionalities, and we suggest these AC-SiNPs as novel promising tools for providing visual evidence of TCM-based cancer treatment.
Similar content being viewed by others
References
Cheung, F. TCM: Made in China. Nature 2011, 480, S82–S83.
Stone, R. Lifting the veil on traditional Chinese medicine. Science 2008, 319, 709–710.
Tsou, L. K.; Lara-Tejero, M.; RoseFigura, J.; Zhang, Z. J.; Wang, Y. C.; Yount, J. S.; Lefebre, M.; Dossa, P. D.; Kato, J.; Guan, F. et al. Antibacterial flavonoids from medicinal plants covalently inactivate type III protein secretion substrates. J. Am. Chem. Soc. 2016, 138, 2209–2218.
Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148.
Koehn, F. E.; Carter, G. T. The evolving role of natural products in drug discovery. Nat. Drug Discov. 2005, 4, 206–220.
Harvey, A. L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901.
Raj, L.; Ide, T.; Gurkar, A. U.; Foley, M.; Schenone, M.; Li, X. Y.; Tolliday, N. J.; Golub, T. R.; Carr, S. A.; Shamji, A. F. et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234.
Gorrini, C.; Harris, I. S.; Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947.
Ostrovsky, S.; Kazimirsky, G.; Gedanken, A.; Brodie, C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009, 2, 882–890.
Kawagishi, H.; Finkel, T. Unraveling the truth about antioxidants: ROS and disease: Finding the right balance. Nat. Med. 2014, 20, 711–713.
Wang, X.; Thormas, B.; Sachdeva, R.; Arterburn, L.; Frye, L; Hatcher, P. G.; Cornwell, D. G.; Ma, J. Mechanism of arylating quinone toxicity involving michael adduct formation and induction of endoplasmic reticulum stress. Proc. Natl. Acad. Sci. USA 2006, 103, 3604–3609.
Hillard, E. A.; de Abreu, F. C.; Ferreira, D. C. M.; Jaouen, G.; Goulart, M. O. F.; Amatore, C. Electrochemical parameters and techniques in drug development, with an emphasis on quinones and related compounds. Chem. Commun. 2008, 2612–2628.
Aziz, M. H.; Dreckschmidt, N. E.; Verma, A. K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032.
Burdick, A. D.; Davis, J. W.; Liu, K. J.; Hudson, L. G.; Shi, H. L.; Monske, M. L.; Burchiei, S. W. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003, 63, 7825–7833.
Tibbitt, M. W.; Dahlman, J. E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717.
Chen, H. W.; Zou, P.; Connarn, J.; Paholak, H.; Sun, D. X. Intracellular dissociation of a polymer coating from nanoparticles. Nano Res. 2012, 5, 815–825.
Mitra, S.; Sasmal, H. S.; Kunda, T.; Kandambeth, S.; Illath, K.; Diaz, D. D.; Banerjee, R. Targeted drug delivery in covalent organic nanosheets (CONs) via sequential postsynthetic modification. J. Am. Chem. Soc. 2017, 139, 4513–4520.
Zheng, F. F.; Zhang, P. H.; Xi, Y.; Chen, J. J.; Li, J. J.; Zhu, J. J. Aptamer/graphene quantum dots nanocomposite capped fluorescent mesoporous silica nanoparticles for intracellular drug delivery and real-time monitoring of drug release. Anal. Chem. 2015, 87, 11739–11745.
Aizil, G.; Waiskopf, N.; Agbaria, M.; Levi-Kalisman, Y.; Banin, U.; Golomb, G. Delivery of liposomal quantum dots via monocytes for imaging of inflamed tissue. ACS Nano 2017, 11, 3038–3051.
Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. J. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120.
He, Y.; Zhong, Y. L.; Peng, F.; Wei, X. P.; Su, Y. Y.; Lu, Y. M.; Su, S.; Gu, W.; Liao, L. S.; Lee, S. T. One-pot microwave synthesis of water-dispersible, ultraphoto-and pH-stable, and highly fluorescent silicon quantum dots. J. Am. Chem. Soc. 2011, 133, 14192–14195.
Zhong, Y. L.; Peng, F.; Wei, X. P.; Zhou, Y. F.; Wang, J.; Jiang, X. X.; Su, Y. Y.; Su, S.; Lee, S. T.; He, Y. Microwave-assisted synthesis of biofunctional and fluorescent silicon nanoparticles using proteins as hydrophilic ligands. Angew. Chem., Int. Ed. 2012, 51, 8485–8489.
Zhong, Y. L.; Peng, F.; Bao, F.; Wang, S. Y.; Ji, X. Y.; Yang, L.; Su, Y. Y.; Lee, S. T.; He, Y. Large-scale aqueous synthesis of fluorescent and biocompatible silicon nanoparticles and their use as highly photostable biological probes. J. Am. Chem. Soc. 2013, 135, 8350–8356.
Wu, S. C.; Zhong, Y. L.; Zhou, Y. F.; Song, B.; Chu, B. B.; Ji, X. Y.; Wu, Y. Y.; Su, Y. Y.; He, Y. Biomimetic preparation and dual-color bioimaging of fluorescent silicon nanoparticles. J. Am. Chem. Soc. 2015, 137, 14726–14732.
Zhong, Y. L.; Sun, X. T.; Wang, S. Y.; Peng, F.; Bao, F.; Su, Y. Y.; Li, Y. Y.; Lee, S. T.; He, Y. Facile, large-quantity synthesis of stable, tunable-color silicon nanoparticles and their application for long-term cellular imaging. ACS Nano 2015, 9, 5958–5967.
Park, J. H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S. N.; Sailor, M. J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336.
Gu, L.; Hall, D. J.; Qin, Z. T.; Anglin, E.; Joo, J.; Mooney, D. J.; Howell, S. B.; Sailor, M. J. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat. Commun. 2013, 4, 2326.
Xing, R.; Li, K. L.; Zhou, Y. F.; Su, Y. Y.; Yan, S. Q.; Zhang, K. L.; Wu, S. C.; Sima, Y. H.; Zhang, K. Q.; He, Y. et al. Impact of fluorescent silicon nanoparticles on circulating hemolymph and hematopoiesis in an invertebrate model organism. Chemosphere 2016, 159, 628–637.
Zhou, Y. F.; Zhang, Y.; Zhong, Y. L.; Fu, R.; Wu, S. C.; Wang, Q.; Wang, H. Y.; Su, Y. Y.; Zhang, H. M.; He, Y. The in vivo targeted molecular imaging of fluorescent silicon nanoparticles in Caenorhabditis Elegans. Nano Res. 2017, in press, DOI: 10.1007/s12274-017-1677-1.
Benezra, M.; Penate-Medina, O.; Zanzonico, P. B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S. et al. Multimodal silica nanoparticles are effective cancertargeted probes in a model of human melanoma. J. Clin. Invest. 2011, 121, 2768–2780.
Phillips, E.; Penate-Medina, O.; Zanzonico, P. B.; Carvajal, R. D.; Möhan, P.; Ye, Y. P.; Humm, J.; Gönen, M.; Kalaigain, H.; Schöder, H.; Strauss, W. H. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Trans. Med. 2014, 6, 260ra149.
Jokerst, J.; Gambhir, S. S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060.
Shiohara, A.; Hanada, S.; Prabakar, S.; Fujioka, K.; Lim, T. H.; Yamamoto, K.; Northcote, P.; Tilley, R. D. Chemical reactions on surface molecules attached to silicon quantum dots. J. Am. Chem. Soc. 2010, 132, 248–253.
Atkins, T. M.; Thibert, A.; Larsen, D. S.; Dey, S.; Browning, N. D.; Kauzlarich, S. M. Femtosecond ligand/core dynamics of microwave-assisted synthesized silicon quantum dots in aqueous solution. J. Am. Chem. Soc. 2011, 133, 20664–20667.
Dasog, M.; Yang, Z. Y.; Regli, S.; Atkins, T. M.; Faramus, A.; Singh, M. P.; Muthuswamy, E.; Kauzlarich, S. M.; Tilley, R. D.; Veinot, J. G. C. Chemical insight into the origin of red and blue photoluminescence arising from freestanding silicon nanocrystals. ACS Nano 2013, 7, 2676–2685.
Ge, M. Y.; Rong, J. P.; Fang, X.; Zhang, A.; Lu, Y. H.; Zhou, C. W. Scalable preparation of porous silicon nanoparticles and their application for lithium-ion battery anodes. Nano Res. 2013, 6, 174–181.
Qian, C. X.; Sun, W.; Wang, L. W.; Chen, C. L.; Liao, K.; Wang, W. D.; Jia, J.; Hatton, B. D.; Casillas, G.; Kurylowicz, M. et al. Non-wettable, oxidation-stable, brightly luminescent, perfluorodecylcapped silicon nanocrystal film. J. Am. Chem. Soc. 2014, 136, 15849–15852.
Kim, T.; Fu, X.; Warther, D.; Sailor, M. J. Size-controlled Pd nanoparticle catalysts prepared by galvanic displacement into a porous Si-iron oxide nanoparticle host. ACS Nano 2017, 11, 2773–2784.
Zhou, T. L.; Anderson, R. T.; Li, H. S.; Bell, J.; Yang, Y. G.; Gorman, B. P.; Pylypenko, S.; Lusk, M. T.; Sellinger, A. Bandgap tuning of silicon quantum dots by surface functionalization with conjugated organic groups. Nano Lett. 2015, 15, 3657–3663.
Cheng, X. Y.; Hinde, E.; Owen, D. M.; Lowe, S. B.; Reece, P. J.; Gaus, K.; Gooding, J. J. Enhancing quantum dots for bioimaging using advanced surface chemistry and advanced optical microscopy: Application to silicon quantum dots (SiQDs). Adv. Mater. 2015, 27, 6144–6150.
Liu, X. K.; Zhang, Y. H.; Yu, T.; Qiao, X. S.; Gresback, R.; Pi, X. D.; Yang, D. R. Optimum quantum yield of the light emission from 2 to 10 nm hydrosilylated silicon quantum dots. Part. Part. Syst. Charact. 2016, 33, 44–52.
Sangghaleh, F.; Sychugov, I.; Yang, Z. Y.; Veinot, J. G. C.; Linnros, J. Near-unity internal quantum efficiency of luminescent silicon nanocrystals with ligand passivation. ACS Nano 2015, 9, 7097–7104.
Erogbogbo, F.; Yong, K. T.; Roy, I.; Xu, G. X.; Prasad, P. N.; Swihart, M. T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878.
Ji, X. Y.; Peng, F.; Zhong, Y. L.; Su, Y. Y.; Jiang, X. X.; Song, C. X.; Yang, L.; Chu, B. B.; Lee, S. T.; He, Y. Highly fluorescent, photostable, and ultrasmall silicon drug nanocarriers for long-term tumor cell tracking and in-vivo cancer therapy. Adv. Mater. 2015, 27, 1029–1034.
Song, C. X.; Zhong, Y. L.; Jiang, X. X.; Peng, F.; Lu, Y. M.; Ji, X. Y.; Su, Y. Y.; He, Y. Peptide-conjugated fluorescent silicon nanoparticles enabling simultaneous tracking and specific destruction of cancer cells. Anal. Chem. 2015, 87, 6718–6723.
Chu, B. B.; Wang, H. Y.; Song, B.; Peng, F.; Su, Y. Y.; He, Y. Fluorescent and photostable silicon nanoparticles sensors for real-time and long-term intracellular pH measurement in live cells. Anal. Chem. 2016, 88, 9235–9242.
Tahover, E.; Patil, Y. P.; Gabizon, A. A. Emerging delivery systems to reduce doxorubicin cardiotoxicity and improve therapeutic index: Focus on liposomes. Anti-Cancer Drugs 2015, 26, 241–258.
Li, X. R.; Yang, X. C.; Lin, Z. Q.; Wang, D.; Mei, D.; He, B.; Wang, X. Y.; Wang, X. Q.; Zhang, Q.; Gao, W. A folate modified pH sensitive targeted polymeric micelle alleviated systemic toxicity of doxorubicin (DOX) in multi-drug resistant tumor bearing mice. Eur. J. Pharm. Sci. 2015, 76, 95–101.
Nitiss, K. C.; Nitiss, J. L. Twisting and ironing: Doxorubicin cardiotoxicity by mitochondrial DNA damage. Clin. Cancer Res. 2014, 20, 4737–4739.
He, Y.; Sai, L. M.; Lu, H. T.; Hu, M.; Lai, W. Y.; Fan, Q. L.; Wang, L. H.; Huang, W. Microwave-assisted synthesis of waterdispersed CdTe nanocrystals with high luminescent efficiency and narrow size distribution. Chem. Mater. 2007, 19, 359–365.
He, Y.; Lu, H. T.; Sai, L. M.; Su, Y. Y.; Hu. M.; Fan, C. H.; Huang, W.; Wang, L. H. Microwave synthesis of waterdispersed CdTe/CdS/ZnS core-shell-shell quantum dots with excellent photostability and biocompatibility. Adv. Mater. 2008, 20, 3416–3421.
Godefroo, S.; Hayne, M.; Jivanescu, M.; Stesmans, A.; Zacharias, M.; Lebedev, O. I.; van Tendeloo, G.; Moshchalkov, V. V. Classification and control of the origin of photoluminescence from Si nanocrystals. Nat. Nanotechnol. 2008, 3, 174–178.
Li, Z. F.; Ruckenstein, E. Water-soluble poly(acrylic acid) grafted luminescent silicon nanoparticles and their use as fluorescent biological staining labels. Nano Lett. 2004, 4, 1463–1467.
Park, J. Y.; Choi, E. S.; Baek, M. J.; Lee, G. H. Colloidal stability of amino acid coated magnetite nanoparticles in physiological fluid. Mater. Lett. 2009, 63, 379–381.
Graf, C.; Gao, Q.; Schütz, I.; Noufele, C. N.; Ruan, W.; Posselt, U.; Korotianskiy, E.; Nordmeyer, D.; Rancan, F.; Hadam, S. et al. Surface functionalization of silica nanoparticles supports colloidal stability in physiological media and facilitates internalization in cells. Langmuir 2012, 28, 7598–7613.
George, P.; Raina, A. K.; Nunomura, A.; Wataya, T.; Sayre, L. M.; Smith, M. A. How important is oxidative damage? Lessons from Alzheimer’s disease. Free Radic. Biol. Med. 2000, 28, 831–834.
Dalle-donne, I.; Rossi, R.; Milzani, A.; Di Simplicid, P.; Colombo, R. The actin cytoskeleton response to oxidants: From small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 2001, 31, 1624–1632.
Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392.
Iyer, A. K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818.
Acknowledgements
We thank Prof. Shuit-Tong Lee (Soochow University, China) for general help and valuable suggestions. We appreciate financial support from the National Basic Research Program of China (No. 2013CB934400), the National Natural Science Foundation of China (Nos. 61361160412, 31400860, 21575096, and 21605109), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), 111 Project as well as Collaborative Innovation Center of Suzhou Nano Science and Technology (NANO-CIC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, X., Guo, D., Song, B. et al. Traditional Chinese medicine molecule-assisted chemical synthesis of fluorescent anti-cancer silicon nanoparticles. Nano Res. 11, 5629–5641 (2018). https://doi.org/10.1007/s12274-018-1976-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-1976-1