Abstract
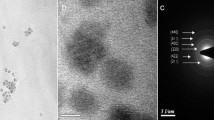
Magnetite nanoparticles (Fe3O4 NPs) are a well proven biocompatible nanomaterial, which hold great promise in various biomedical applications. Interestingly, unlike conventional biocompatible materials (e.g., polyethylene glycol (PEG)) that are chemically and biologically inert in nature, Fe3O4 NPs are known to be catalytically active and exhibit prominent physiological effects. Herein, we report an “active”, dynamic equilibrium mechanism for maintaining the cellular amenity of Fe3O4 NPs. We examined the effects of two types of iron oxide (magnetite and hematite) NPs in rat pheochromocytoma (PC12) cells and found that both induced stress responses. However, only Fe2O3 NPs caused significant programmed cell death; whereas Fe3O4 NPs are amenable to cells. We found that intrinsic catalase-like activity of Fe3O4 NPs antagonized the accumulation of toxic reactive oxygen species (ROS) induced by themselves, and thereby modulated the extent of cellular oxidative stress, autophagic activity, and programmed cell death. In line with this observation, we effectively reversed severe autophagy and cell death caused by Fe2O3 NPs via co-treatment with natural catalase. This study not only deciphers the distinct intrinsic antagonism of Fe3O4 NPs, but opens new routes to designing biocompatible theranostic nanoparticles with novel mechanisms.

Similar content being viewed by others
References
Krug, H. F. Nanosafety research—Are we on the right track? Angew. Chem., Int. Ed. 2014, 53, 12304–12319.
Yu, Y.; Cui, C.; Liu, X. H.; Petrik, I. D.; Wang, J. Y.; Lu, Y. A designed metalloenzyme achieving the catalytic rate of a native enzyme. J. Am. Chem. Soc. 2015, 137, 11570–11573.
Zhao, Z.; Fu, J. L.; Dhakal, S.; JohnsonBuck, A.; Liu, M. H.; Zhang, T.; Woodbury, N. W.; Liu, Y.; Walter, N. G.; Yan, H. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat. Commun. 2016, 7, 10619.
Liu, J. L.; Yang, Y.; Zhu, W. W.; Yi, X.; Dong, Z. L.; Xu, X. N.; Chen, M. W.; Yang, K.; Lu, G.; Jiang, L. et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9.
Li, M. Y.; Li, L. L.; Zhan, C. Y.; Kohane, D. S. Core–shell nanostars for multimodal therapy and imaging. Theranostics 2016, 6, 2306–2313.
Xu, M.; Zhu, J. Q.; Wang, F. F.; Xiong, Y. J.; Wu, Y. K.; Wang, Q. Q.; Weng, J.; Zhang, Z. H.; Chen, W.; Liu, S. J. Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly(acrylic acid)-functionalization is superior to pegylation. ACS Nano 2016, 10, 3267–3281.
Li, Y. J.; Feng, L. Z.; Shi, X. Z.; Wang, X. J.; Yang, Y. L.; Yang, K.; Liu, T.; Yang, G. B.; Liu, Z. Surface coatingdependent cytotoxicity and degradation of graphene derivatives: Towards the design of non-toxic, degradable nano-graphene. Small 2014, 10, 1544–1554.
Liu, Q.; Wang, W. P.; Zhan, C. Y.; Yang, T. S.; Kohane, D. S. Enhanced precision of nanoparticle phototargeting in vivo at a safe irradiance. Nano Lett. 2016, 16, 4516–4520.
Lee, D. H.; Kang, M.; Lee, H. J.; Kim, J. A.; Choi, Y. K.; Cho, H.; Park, J. K.; Park, T. H.; Jung, H. Enhanced cellular uptake of silica-coated magnetite nanoparticles compared with peg-coated ones in stem cells. J. Nanosci. Nanotechnol. 2015, 15, 5512–5519.
Walczyk, D.; Bombelli, F. B.; Monopoli, M. P.; Lynch, I.; Dawson, K. A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768.
Shi, M.; Cheng, L.; Zhang, Z. B.; Liu, Z.; Mao, X. L. Ferroferric oxide nanoparticles induce prosurvival autophagy in human blood cells by modulating the beclin 1/Bcl-2/ VPS34 complex. Int. J. Nanomedicine 2015, 10, 207–216.
Chen, N.; Wang, H.; Huang, Q.; Li, J.; Yan, J.; He, D. N.; Fan, C. H.; Song, H. Y. Long-term effects of nanoparticles on nutrition and metabolism. Small 2014, 10, 3603–3611.
Vernet, E.; Popa, G.; Pozdnyakova, I.; Rasmussen, J. E.; Grohganz, H.; Giehm, L.; Jensen, M. H.; Wang, H. B.; Plesner, B.; Nielsen, H. M. et al. Large-scale biophysical evaluation of protein pegylation effects: In vitro properties of 61 protein entities. Mol. Pharm. 2016, 13, 1587–1598.
Harrison, E.; Coulter, J. A.; Dixon, D. Gold nanoparticle surface functionalization: Mixed monolayer versus hetero bifunctional peg linker. Nanomedicine 2016, 11, 851–865.
Lee, N.; Yoo, D.; Ling, D. S.; Cho, M. H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689.
Yoo, D.; Lee, J. H.; Shin, T. H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874.
Colombo, M.; Carregal-Romero, S.; Casula, M. F.; Gutierrez, L.; Morales, M. P.; Böhm, I. B.; Heverhagen, J. T.; Prosperi, D.; Parak, W. J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334.
Kong, S. D.; Lee, J.; Ramachandran, S.; Eliceiri, B. P.; Shubayev, V. I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control Release 2012, 164, 49–57.
Yi, P. W.; Chen, G. C.; Zhang, H. L.; Tian, F.; Tan, B.; Dai, J. W.; Wang, Q. B.; Deng, Z. W. Magnetic resonance imaging of Fe3O4@SiO2-labeled human mesenchymal stem cells in mice at 11.7 T. Biomaterials 2013, 34, 3010–3019.
Mo, A. H.; Landon, P. B.; Gomez, K. S.; Kang, H.; Lee, J.; Zhang, C.; Janetanakit, W.; Sant, V.; Lu, T. Y.; Colburn, D. A. et al. Magnetically-responsive silica-gold nanobowls for targeted delivery and sers-based sensing. Nanoscale 2016, 8, 11840–11850.
Han, J.; Kim, B.; Shin, J. Y.; Ryu, S.; Noh, M.; Woo, J.; Park, J. S.; Lee, Y.; Lee, N.; Hyeon, T. et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015, 9, 2805–2819.
Ling, D. S.; Lee, N.; Hyeon, T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc. Chem. Res. 2015, 48, 1276–1285.
Gao, L. Z.; Zhuang, J.; Nie, L.; Zhang, J. B.; Zhang, Y.; Gu, N.; Wang, T. H.; Feng, J.; Yang, D. L.; Perrett, S. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583.
Chen, Z. E.; Yin, J. J.; Zhou, Y. T.; Zhang, Y.; Song, L. N.; Song, M. J.; Hu, S. L.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012.
Zhang, Y.; Wang, Z. Y.; Li, X. J.; Wang, L.; Yin, M.; Wang, L. H.; Chen, N.; Fan, C. H.; Song, H. Y. Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in drosophila. Adv. Mater. 2016, 28, 1387–1393.
Zhong, W. Y.; Lü, M.; Liu, L. Y.; Sun, J. L.; Zhong, Z. T.; Zhao, Y.; Song, H. Y. Autophagy as new emerging cellular effect of nanomaterials. Chin. Sci. Bull. 2013, 58, 4031–4038.
Peynshaert, K.; Manshian, B. B.; Joris, F.; Braeckmans, K.; De Smedt, S. C.; Demeester, J.; Soenen, S. J. Exploiting intrinsic nanoparticle toxicity: The pros and cons of nanoparticle-induced autophagy in biomedical research. Chem. Rev. 2014, 114, 7581–7609.
Popp, L.; Segatori, L. Differential autophagic responses to nano-sized materials. Curr. Opin. Biotechnol. 2015, 36, 129–136.
Wan, B.; Wang, Z. X.; Lv, Q. Y.; Dong, P. X.; Zhao, L. X.; Yang, Y.; Guo, L. H. Single-walled carbon nanotubes and graphene oxides induce autophagosome accumulation and lysosome impairment in primarily cultured murine peritoneal macrophages. Toxicol. Lett. 2013, 221, 118–127.
Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of atg4. EMBO J. 2007, 26, 1749–1760.
Luo, Y. H.; Wu, S. B.; Wei, Y. H.; Chen, Y. C.; Tsai, M. H.; Ho, C. C.; Lin, S. Y.; Yang, C. S.; Lin, P. P. Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem. Res. Toxicol. 2013, 26, 662–673.
Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49.
Chen, N.; He, Y.; Su, Y. Y.; Li, X. M.; Huang, Q.; Wang, H. F.; Zhang, X. Z.; Tai, R. Z.; Fan, C. H. The cytotoxicity of cadmium-based quantum dots. Biomaterials 2012, 33, 1238–1244.
Wang, B.; Chen, N.; Wei, Y. L.; Li, J.; Sun, L.; Wu, J. R.; Huang, Q.; Liu, C.; Fan, C. H.; Song, H. Y. Akt signalingassociated metabolic effects of dietary gold nanoparticles in drosophila. Sci. Rep. 2012, 2, 563.
Ma, P. A.; Xiao, H. H.; Yu, C.; Liu, J. H.; Cheng, Z. Y.; Song, H. Q.; Zhang, X. Y.; Li, C. X.; Wang, J. Q.; Gu, Z. et al. Enhanced cisplatin chemotherapy by iron oxide nanocarriermediated generation of highly toxic reactive oxygen species. Nano Lett. 2017, 17, 928–937.
Scherz-Shouval, R.; Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007, 17, 422–427.
Li, C. G.; Liu, H. L.; Sun, Y.; Wang, H. L.; Guo, F.; Rao, S.; Deng, J. J.; Zhang, Y. L.; Miao, Y. F.; Guo, C. Y. et al. Pamam nanoparticles promote acute lung injury by inducing autophagic cell death through the akt-tsc2-mtor signaling pathway. J. Mol. Cell. Biol. 2009, 1, 37–45.
Guan, Y. J.; Li, M.; Dong, K.; Gao, N.; Ren, J. S.; Zheng, Y. C.; Qu, X. G. Ceria/poms hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-beta peptide. Biomaterials 2016, 98, 92–102.
White, E.; DiPaola, R. S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 2009, 15, 5308–5316.
Levine, B.; Mizushima, N.; Virgin, H. W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335.
Mariño, G.; Niso-Santano, M.; Baehrecke, E. H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 81–94.
Booth, L. A.; Tavallai, S.; Hamed, H. A.; Cruickshanks, N.; Dent, P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell. Signal. 2014, 26, 549–555.
Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S. J.; Gibson, S. B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182.
Khan, M. I.; Mohammad, A.; Patil, G.; Naqvi, S. A. H.; Chauhan, L. K. S.; Ahmad, I. Induction of ros, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488.
Acknowledgements
We would like to dedicate this article to Professor Qing Huang. This work was supported by National Natural Science Foundation of China (Nos. 31771102, 31371015, 21675167, U1532119, 31470970, 31371493, and 31571498), the National Basic Research Program of China (Nos. 2013CB932803, 2013CB933802, 2016YFA0400900, and 2016YFA0201200), the Youth Innovation Promotion Association from Chinese Academy of Sciences (No. 2015211), Key Research Program of Frontier Sciences, CAS (Nos. QYZDJ-SSW-SLH019 and QYZDJ-SSW-SLH031).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, L., Wang, Z., Li, X. et al. Deciphering active biocompatibility of iron oxide nanoparticles from their intrinsic antagonism. Nano Res. 11, 2746–2755 (2018). https://doi.org/10.1007/s12274-017-1905-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1905-8