Abstract
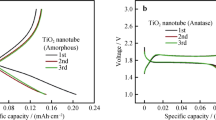
Due to their inherent safety, low cost, and structural stability, TiO2 nanostructures represent a suitable choice as anode materials in sodium-ion batteries. In the recent years, various hypotheses have been proposed regarding the actual mechanism of the reversible insertion of sodium ions in the TiO2 structure, and previous reports are often controversial in this respect. Interestingly, when tested as binder- and conducting additive-free electrodes in laboratory-scale sodium cells, amorphous and crystalline (anatase) TiO2 nanotubular arrays obtained by simple anodic oxidation exhibit peculiar and intrinsically different electrochemical responses. In particular, after the initial electrochemical activation, anatase TiO2 shows excellent rate capability and very stable long-term cycling performance with larger specific capacities, and thus a clearly superior response compared with the amorphous counterpart. To obtain deeper insight, the present materials are thoroughly characterized by scanning electron microscopy and ex situ X-ray diffraction, and the insertion of sodium ions in the TiO2 bulk phases is systematically modeled by density functional theory calculations. The present results may contribute to the development of more systematic screening approaches to identify suitable active materials for highly efficient sodium-based energy storage systems.

Similar content being viewed by others
References
Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium dioxide (anatase and rutile): Surface chemistry, liquid-solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114, 9754–9823.
Chen, X. B.; Liu, L.; Huang, F. Q. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885.
Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454.
Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130.
Zhao, Y.; Hoivik, N.; Wang, K. Y. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744.
Ansari, S. A.; Khan, M. M.; Ansari, M. O.; Cho, M. H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009.
Abdullah, N.; Kamarudin, S. K. Titanium dioxide in fuel cell technology: An overview. J. Power Sources 2015, 278, 109–118.
Ma, Y.; Wang, X. L.; Jia, Y. S.; Chen, X. B.; Han, H. X.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043.
Wang, X. D.; Li, Z. D.; Shi, J.; Yu, Y. H. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 2014, 114, 9346–9384.
Zhao, F. P.; Gong, Q. F.; Traynor, B.; Zhang, D.; Li, J. J.; Ye, H. L.; Chen, F. J.; Han, N.; Wang, Y. Y.; Sun, X. H. et al. Stabilizing nickel sulfide nanoparticles with an ultrathin carbon layer for improved cycling performance in sodium ion batteries. Nano Res. 2016, 9, 3162–3170.
Shoaib, A.; Huang, Y. X.; Liu, J.; Liu, J. J.; Xu, M.; Wang, Z. H.; Chen, R. J.; Zhang, J. T.; Wu, F. Ultrathin singlecrystalline TiO2 nanosheets anchored on graphene to be hybrid network for high-rate and long cycle-life sodium battery electrode application. J. Power Sources 2017, 342, 405–413.
Zhou, M.; Xu, Y.; Wang, C. L.; Li, Q. W.; Xiang, J. X.; Liang, L. Y.; Wu, M. H.; Zhao, H. P.; Lei, Y. Amorphous TiO2 inverse opal anode for high-rate sodium ion batteries. Nano Energy 2017, 31, 514–524.
Chen, J.; Zhang, Y.; Zou, G. Q.; Huang, Z. D.; Li, S. M.; Liao, H. X.; Wang, J. F.; Hou, H. S.; Ji, X. B. Size-tunable olive-like anatase TiO2 coated with carbon as superior anode for sodium-ion batteries. Small 2016, 12, 5554–5563.
Song, H.; Jo, K.; Jung, B. Y.; Jung, G. Y. Fabrication of periodically aligned vertical single-crystalline anatase TiO2 nanotubes with perfect hexagonal open-ends using chemical capping materials. Nano Res. 2014, 7, 104–109.
Wang, B. F.; Zhao, F.; Du, G. D.; Porter, S.; Liu, Y.; Zhang, P.; Cheng, Z. X.; Liu, H. K.; Huang, Z. G. Boron-doped anatase TiO2 as a high-performance anode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 16009–16015.
Lee, J.; Lee, J. K.; Chung, K. Y.; Jung, H. G.; Kim, H.; Mun, J.; Choi, W. Electrochemical investigations on TiO2-B nanowires as a promising high capacity anode for sodiumion batteries. Electrochim. Acta 2016, 200, 21–28.
Das, S. K.; Jache, B.; Lahon, H.; Bender, C. L.; Janek, J.; Adelhelm, P. Graphene mediated improved sodium storage in nanocrystalline anatase TiO2 for sodium ion batteries with ether electrolyte. Chem. Commun. 2016, 52, 1428–1431.
Xiong, Y.; Qian, J. F.; Cao, Y. L.; Ai, X. P.; Yang, H. X. Graphene-supported TiO2 nanospheres as a high-capacity and long-cycle life anode for sodium ion batteries. J. Mater. Chem. A 2016, 4, 11351–11356.
Xiong, H.; Slater, M. D.; Balasubramanian, M.; Johnson, C. S.; Rajh, T. Amorphous TiO2 nanotube anode for rechargeable sodium ion batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565.
Pintossi, D.; Iannaccone, G.; Colombo, A.; Bella, F.; Välimäki, M.; Väisänen, K. L.; Hast, J.; Levi, M.; Gerbaldi, C.; Dragonetti, C.et al. Luminescent downshifting by photoinduced sol-gel hybrid coatings: Accessing multifunctionality on flexible organic photovoltaics via ambient temperature material processing. Adv. Electron. Mater. 2016, 2, 1600288.
Bella, F.; Galliano, S.; Falco, M.; Viscardi, G.; Barolo, C.; Grätzel, M.; Gerbaldi, C. Approaching truly sustainable solar cells by the use of water and cellulose derivatives. Green Chem. 2017, 19, 1043–1051.
Bella, F.; Chiappone, A.; Nair, J. R.; Meligrana, G.; Gerbaldi, C. Effect of different green cellulosic matrices on the performance of polymeric dye-sensitized solar cells. Chem. Eng. Trans. 2014, 41, 211–216.
Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682.
Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L. F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem., Int. Ed. 2015, 54, 3431–3448.
Wang, L. P.; Yu, L. H.; Wang, X.; Srinivasan, M.; Xu, Z. J. Recent developments in electrode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 9353–9378.
Li, H. B.; Zhou, Q. Y.; Gao, Y. T.; Gui, X. C.; Yang, L.; Du, M. D.; Shi, E. Z.; Shi, J. D.; Cao, A. Y.; Fang, Y. Templated synthesis of TiO2 nanotube macrostructures and their photocatalytic properties. Nano Res. 2015, 8, 900–906.
Zhang, N.; Liu, Y. C.; Lu, Y. Y.; Han, X. P.; Cheng, F. Y.; Chen, J. Spherical nano-Sb@C composite as a high-rate and ultra-stable anode material for sodium-ion batteries. Nano Res. 2015, 8, 3384–3393.
Xu, Y.; Zhou, M.; Lei, Y. Nanoarchitectured array electrodes for rechargeable lithium- and sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1502514.
Su, H.; Jaffer, S.; Yu, H. J. Transition metal oxides for sodium-ion batteries. Energy Storage Mater. 2016, 5, 116–131.
Guo, S. H.; Yi, J.; Sun, Y.; Zhou, H. S. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries. Energy Environ. Sci. 2016, 9, 2978–3006.
Kim, H.; Kim, H.; Ding, Z.; Lee, M. H.; Lim, K.; Yoon, G.; Kang, K. Recent progress in electrode materials for sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600943.
Kim, H.; Yoon, G.; Park, I.; Hong, J.; Park, K. Y.; Kim, J.; Lee, K. S.; Sung, N. E.; Lee, S.; Kang, K. Highly stable ironand manganese-based cathodes for long-lasting sodium rechargeable batteries. Chem. Mater. 2016, 28, 7241–7249.
Tsuchiya, Y.; Takanashi, K.; Nishinobo, T.; Hokura, A.; Yonemura, M.; Matsukawa, T.; Ishigaki, T.; Yamanaka, K.; Ohta, T.; Yabuuchi, N. Layered NaxCrxTi1–xO2 as bifunctional electrode materials for rechargeable sodium batteries. Chem. Mater. 2016, 28, 7006–7016.
Cha, H. A.; Jeong, H. M.; Kang, J. K. Nitrogen-doped open pore channeled graphene facilitating electrochemical performance of TiO2 nanoparticles as an anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 5182–5186.
Gao, H.; Zhou, T. F.; Zheng, Y.; Liu, Y. Q.; Chen, J.; Liu, H. K.; Guo, Z. P. Integrated carbon/red phosphorus/ graphene aerogel 3D architecture via advanced vaporredistribution for high-energy sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1601037.
Wang, X. F.; Li, Y. J.; Gao, Y. R.; Wang, Z. X.; Chen, L. Q. Additive-free sodium titanate nanotube array as advanced electrode for sodium ion batteries. Nano Energy 2015, 13, 687–692.
Wu, L. M.; Bresser, D.; Buchholz, D.; Giffin, G. A.; Castro, C. R.; Ochel, A.; Passerini, S. Unfolding the mechanism of sodium insertion in anatase TiO2 nanoparticles. Adv. Energy Mater. 2015, 5, 1401142.
Zhou, M.; Xu, Y.; Xiang, J. X.; Wang, C. L.; Liang, L. Y.; Wen, L. Y.; Fang, Y. G.; Mi, Y.; Lei, Y. Understanding the orderliness of atomic arrangement toward enhanced sodium storage. Adv. Energy Mater. 2016, 6, 1600448.
Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem., Int. Ed. 2011, 50, 2904–2939.
Ortiz, G. F.; Hanzu, I.; Djenizian, T.; Lavela, P.; Tirado, J. L.; Knauth, P. Alternative Li-ion battery electrode based on selforganized titania nanotubes. Chem. Mater. 2009, 21, 63–67.
González, J. R.; Alcántara, R.; Ortiz, G. F.; Nacimiento, F.; Tirado, J. L. Controlled growth and application in lithium and sodium batteries of high-aspect-ratio, self-organized titania nanotubes. J. Electrochem. Soc. 2013, 160, A1390–A1398.
Zhu, K.; Neale, N. R.; Miedaner, A.; Frank, A. J. Enhanced charge-collection efficiencies and light scattering in dyesensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74.
Wu, L. M.; Buchholz, D.; Bresser, D.; Chagas, L. G.; Passerini, S. Anatase TiO2 nanoparticles for high power sodium-ion anodes. J. Power Sources 2014, 251, 379–385.
González, J. R.; Alcántara, R.; Nacimiento, F.; Ortiz, G. F.; Tirado, J. L. Self-organized, anatase, double-walled nanotubes prepared by anodization under voltage ramp as negative electrode for aqueous sodium-ion batteries. J. Electrochem. Soc. 2015, 162, A3007–A3012.
Weadock, N.; Varongchayakul, N.; Wan, J. Y.; Lee, S.; Seog, J.; Hu, L. B. Determination of mechanical properties of the SEI in sodium ion batteries via colloidal probe microscopy. Nano Energy 2013, 2, 713–719.
Lamberti, A.; Garino, N.; Sacco, A.; Bianco, S.; Chiodoni, A.; Gerbaldi, C. As-grown vertically aligned amorphous TiO2 nanotube arrays as high-rate Li-based micro-battery anodes with improved long-term performance. Electrochim. Acta 2015, 151, 222–229.
Yan, D.; Yu, C. Y.; Bai, Y.; Zhang, W. F.; Chen, T. Q.; Hu, B. W.; Sun, Z.; Pan, L. K. Sn-doped TiO2 nanotubes as superior anode materials for sodium ion batteries. Chem. Commun. 2015, 51, 8261–8264.
Lamberti, A.; Garino, N.; Sacco, A.; Bianco, S.; Manfredi, D.; Gerbaldi, C. Vertically aligned TiO2 nanotube array for high rate Li-based micro-battery anodes with improved durability. Electrochim. Acta 2013, 102, 233–239.
Portenkirchner, E.; Neri, G.; Lichtinger, J.; Brumbarov, J.; Rüdiger, C.; Gernhäuser, R.; Kunze-Liebhäuser, J. Tracking areal lithium densities from neutron activation–quantitative Li determination in self-organized TiO2 nanotube anode materials for Li-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 8602–8611.
Prasai, B.; Cai, B.; Underwood, M. K.; Lewis, J. P.; Drabold, D. A. Properties of amorphous and crystalline titanium dioxide from first principles. J. Mater. Sci. 2012, 47, 7515–7521.
Legrain, F.; Malyi, O.; Manzhos, S. Insertion energetics of lithium, sodium, and magnesium in crystalline and amorphous titanium dioxide: A comparative first-principles study. J. Power Sources 2015, 278, 197–202.
Anwar, T.; Wang, L.; Sagar, R. U. R.; Nosheen, F.; Singh, R.; Jafri, H. M.; Shehzad, K.; Liang, T. X. Cathodic titania nanotube arrays as anode material for lithium-ion batteries. J. Mater. Sci. 2017, 52, 4323–4332.
El-Nahrawy, A. M.; Ali, A. I.; Hammad, A. B. A.; Youssef, A. M. Influences of Ag-NPs doping chitosan/calcium silicate nanocomposites for optical and antibacterial activity. Int. J. Biol. Macromol. 2016, 93, 267–275.
Lu, Y.; Wang, T. Y.; Li, X. R.; Zhang, G. X.; Xue, H. G.; Pang, H. Synthetic methods and electrochemical applications for transition metal phosphide nanomaterials. RSC Adv. 2016, 6, 87188–87212.
Hou, G. M.; Zhang, M. Q.; Huang, Y. F.; Ruan, W. H. A TiO2/PEO composite incorporated with in situ synthesized hyper-branched poly(amine-ester) and its application as a polymer electrolyte. RSC Adv. 2016, 6, 83406–83411.
Zhou, X.; Obadia, M. M.; Venna, S. R.; Roth, E. A.; Serghei, A.; Luebke, D. R.; Myers, C.; Chang, Z. M.; Enick, R.; Drockenmuller, E. et al. Highly cross-linked polyether-based 1,2,3-triazolium ion conducting membranes with enhanced gas separation properties. Eur. Polym. J. 2016, 84, 65–76.
Xu, S. S.; Wang, Y. H.; Zhao, Y.; Chen, W. L.; Wang, J. B.; He, L. F.; Su, Z. M.; Wang, E. B.; Kang, Z. H. Kepleratetype polyoxometalate/semiconductor composite electrodes with light-enhanced conductivity towards highly efficient photoelectronic devices. J. Mater. Chem. A 2016, 4, 14025–14032.
Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J. R. Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci. Rep. 2016, 6, 19892.
Salvador, G. P.; Pugliese, D.; Bella, F.; Chiappone, A.; Sacco, A.; Bianco, S.; Quaglio, M. New insights in long-term photovoltaic performance characterization of cellulosebased gel electrolytes for stable dye-sensitized solar cells. Electrochim. Acta 2014, 146, 44‒51.
Nair, J. R.; Porcarelli, L.; Bella, F.; Gerbaldi, C. Newly elaborated multipurpose polymer electrolyte encompassing RTILs for smart energy-efficient devices. ACS Appl. Mater. Interfaces 2015, 7, 12961‒12971.
Bella, F.; Sacco, A.; Massaglia, G.; Chiodoni, A.; Pirri, C. F.; Quaglio, M. Dispelling clichés at the nanoscale: the true effect of polymer electrolytes on the performance of dye-sensitized solar cells. Nanoscale 2015, 7, 12010–12017.
Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871.
Kohn, W.; Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Ponti, G.; Palombi, F.; Abate, D.; Ambrosino, F.; Aprea, G.; Bastianelli, T.; Beone, F.; Bertini, R.; Bracco, G.; Caporicci, M. et al. The role of medium size facilities in the HPC ecosystem: the case of the new CRESCO4 cluster integrated in the ENEAGRID infrastructure. In Proceedings of the 2014 International Conference on High Performance Computing and Simulation, Bologna, Italy, 2014, pp 1030–1033.
Acknowledgements
Authors would like to thank Mr. Mauro Raimondo for the surface and cross-sectional FESEM/EDX analysis of the as-prepared samples. Prof. Drabold D. A. is kindly acknowledged for providing the amorphous TiO2 coordinates. The computing resources and the related technical support used for this work have been provided by CRESCO/ENEAGRID High Performance Computing infrastructure and its staff [70]. CRESCO/ ENEAGRID High Performance Computing infrastructure is funded by ENEA, the Italian National Agency for New Technologies, Energy and Sustainable Economic Development and by Italian and European research programs, see http://www.cresco.enea.it/english for information. Part of this work is carried out within the activities “Ricerca Sistema Elettrico” funded through contributions to research and development by the Italian Ministry of Economic Development.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bella, F., Muñoz-García, A.B., Meligrana, G. et al. Unveiling the controversial mechanism of reversible Na storage in TiO2 nanotube arrays: Amorphous versus anatase TiO2 . Nano Res. 10, 2891–2903 (2017). https://doi.org/10.1007/s12274-017-1656-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1656-6