Abstract
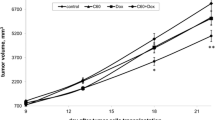
We formulated and analyzed a novel nanoformulation of the anticancer drug cisplatin (Cis) with C60 fullerene (C60+Cis complex) and showed its higher toxicity toward tumor cell lines in vitro when compared to Cis alone. The highest toxicity of the complex was observed in HL-60/adr and HL-60/vinc chemotherapy-resistant human leukemia cell sublines (resistant to Adriamycin and Vinculin, respectively). We discovered that the action of the C60+Cis complex is associated with overcoming the drug resistance of the tumor cell lines through observing an increased number of apoptotic cells in the Annexin V/PI assay. Moreover, in vivo assays with Lewis lung carcinoma (LLC) C57BL/6J male mice showed that the C60+Cis complex increases tumor growth inhibition, when compared to Cis or C60 fullerenes alone. Simultaneously, we conducted a molecular docking study and performed an Ames test. Molecular docking specifies the capability of a C60 fullerene to form van der Waals interactions with potential binding sites on P-glycoprotein (P-gp), multidrug resistance protein 1 (MRP-1), and multidrug resistance protein 2 (MRP-2) molecules. The observed phenomenon revealed a possible mechanism to bypass tumor cell drug resistance by the C60+Cis complex. Additionally, the results of the Ames test show that the formation of such a complex diminishes the Cis mutagenic activity and may reduce the probability of secondary neoplasm formation. In conclusion, the C60+Cis complex effectively induced tumor cell death in vitro and inhibited tumor growth in vivo, overcoming drug resistance likely by the potential of the C60 fullerene to interact with P-gp, MRP-1, and MRP-2 molecules. Thus, the C60+Cis complex might be a potential novel chemotherapy modification.

Similar content being viewed by others
References
Corrie, P. G. Cytotoxic chemotherapy: Clinical aspects. Medicine 2008, 36, 24–28.
de Vita, V. T.; Hellman, S.; Rosenberg, S. A. Principles and Practice of Oncology; 6th ed.; Lippincott, Williams & Wilkins: Philadelphia, 2001.
Hirsch, J. An anniversary for cancer chemotherapy. JAMA 2006, 296, 1518–1520.
Florea, A. M.; Busselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011, 3, 1351–1371.
Huynh, V. T.; Scarano, W.; Stenzel, M. H. Drug delivery systems for platinum drugs. In Nanopharmaceutics. Liang, X. J., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2012; pp 201–241.
Carmona, R.; Liang, X.-J. Improving platinum efficiency: Nanoformulations. In Nanopharmaceutics. Liang, X. J., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2012; pp 243–274.
Liu, L.; Ye, Q.; Lu, M.; Lo, Y. C.; Hsu, Y. H.; Wei, M. C.; Chen, Y. H.; Lo, S. C.; Wang, S. J.; Bain, D. J. et al. A new approach to reduce toxicities and to improve bioavailabilities of platinum-containing anti-cancer nanodrugs. Sci. Rep. 2015, 5, 10881.
Dong, X. P.; Xiao, T. H.; Dong, H.; Jiang, N.; Zhao, X. G. Endostar combined with cisplatin inhibits tumor growth and lymphatic metastasis of Lewis lung carcinoma xenografts in mice. Asian Pac. J. Cancer Prev. 2013, 14, 3079–3083.
Yu, H. Y.; Tang, Z. H.; Li, M. Q.; Song, W. T.; Zhang, D. W.; Zhang, Y.; Yang, Y.; Sun, H.; Deng, M. X.; Chen, X. S. Cisplatin loaded poly(L-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for potential cancer therapy: Preparation, in vitro and in vivo evaluation. J. Biomed. Nanotechnol. 2016, 12, 69–78.
Cataldo, F.; Da Ros, T. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Springer: Amsterdam, 2008.
Andrievsky, G.; Klochkov, V.; Derevyanchenko, L. Is the C60 fullerene molecule toxic?! Fullerenes, Nanotubes, Carbon Nanostruct. 2005, 13, 363–376.
Prylutska, S. V.; Matyshevska, O. P.; Golub, A. A.; Prylutskyy, Y. I.; Potebnya, G. P.; Ritter, U.; Scharff, P. Study of C60 fullerenes and C60-containing composites cytotoxicity in vitro. Mater. Sci. Eng. C 2007, 27, 1121–1124.
Johnston, H. J.; Hutchinson, G. R.; Christensen, F. M.; Aschberger, K.; Stone, V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol. Sci. 2010, 114, 162–182.
Prylutska, S.; Bilyy, R.; Overchuk, M.; Bychko, A.; Andreichenko, K.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N. G.; Ritter, U. Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J. Biomed. Nanotechnol. 2012, 8, 522–527.
Bedrov, D.; Smith, G. D.; Davande, H.; Li, L. W. Passive transport of C60 fullerenes through a lipid membrane: A molecular dynamics simulation study. J. Phys. Chem. B 2008, 112, 2078–2084.
Qiao, R.; Roberts, A. P.; Mount, A. S.; Klaine, S. J.; Ke, P. C. Translocation of C60 and its derivatives across a lipid bilayer. Nano Lett. 2007, 7, 614–619.
Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S. R.; Moussa, F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585.
Prylutska, S. V.; Grynyuk, I. I.; Matyshevska, O. P.; Prylutskyy, Y. I.; Ritter, U.; Scharff, P. Anti-oxidant properties of C60 fullerenes in vitro. Fullerenes, Nanotubes, Carbon Nanostruct. 2008, 16, 698–705.
Prylutska, S. V.; Burlaka, A. P.; Klymenko, P. P.; Grynyuk, I. I.; Prylutskyy, Y. I.; Schü tze, C.; Ritter, U. Using water-soluble C60 fullerenes in anticancer therapy. Cancer Nanotechnol. 2011, 2, 105–110.
Prylutska, S. V.; Burlaka, A. P.; Prylutskyy, Y. I.; Ritter, U.; Scharff, P. Pristine C(60) fullerenes inhibit the rate of tumor growth and metastasis. Exp. Oncol. 2011, 33, 162–164.
Chen, Z. Y.; Ma, L. J.; Liu, Y.; Chen, C. Y. Applications of functionalized fullerenes in tumor theranostics. Theranostics 2012, 2, 238–250.
Didenko, G.; Prylutska, S.; Kichmarenko, Y.; Potebnya, G.; Prylutskyy, Y.; Slobodyanik, N.; Ritter, U.; Scharff, P. Evaluation of the antitumor immune response to C60 fullerene. Materialwiss. Werkstofftech. 2013, 44, 124–128.
Kato, S.; Aoshima, H.; Saitoh, Y.; Miwa, N. Fullerene-C60 derivatives prevent UV-irradiation/TiO2-induced cytotoxicity on keratinocytes and 3D-skin tissues through antioxidant actions. J. Nanosci. Nanotechnol. 2014, 14, 3285–3291.
Bozdaganyan, M. E.; Orekhov, P. S.; Shaytan, A. K.; Shaitan, K. V. Comparative computational study of interaction of C60-fullerene and tris-malonyl-C60-fullerene isomers with lipid bilayer: Relation to their antioxidant effect. PLoS One 2014, 9, e102487.
Zhu, J. D.; Ji, Z. Q.; Wang, J.; Sun, R. H.; Zhang, X.; Gao, Y.; Sun, H. F.; Liu, Y. F.; Wang, Z.; Li, A. D. et al. Tumorinhibitory effect and immunomodulatory activity of fullerol C60(OH)x. Small 2008, 4, 1168–1175.
Evstigneev, M. P.; Buchelnikov, A. S.; Voronin, D. P.; Rubin, Y. V.; Belous, L. F.; Prylutskyy, Y. I.; Ritter, U. Complexation of C60 fullerene with aromatic drugs. ChemPhysChem 2013, 14, 568–578.
Prylutskyy, Y. I.; Evstigneev, M. P.; Pashkova, I. S.; Wyrzykowski, D.; Woziwodzka, A.; Golunski, G.; Piosik, J.; Cherepanov, V. V.; Ritter, U. Characterization of C60 fullerene complexation with antibiotic doxorubicin. Phys. Chem. Chem. Phys. 2014, 16, 23164–23172.
Prylutska, S. V.; Didenko, G. V.; Potebnya, G. P.; Bogutska, K. I.; Prylutskyy, Y. I.; Ritter, U.; Scharff, P. Toxic effect of C60 fullerene-doxorubicin complex towards tumor and normal cells in vitro. Biopolym. Cell 2014, 30, 372–376.
Panchuk, R. R.; Prylutska, S. V.; Chumak, V. V.; Skorokhyd, N. R.; Lehka, L. V.; Evstigneev, M. P.; Prylutskyy, Y. I.; Berger, W.; Heffter, P.; Scharff, P. et al. Application of C60 fullerene-doxorubicin complex for tumor cell treatment in vitro and in vivo. J. Biomed. Nanotechnol. 2015, 11, 1139–1152.
Prylutska, S. V.; Skivka, L. M.; Didenko, G. V.; Prylutskyy, Y. I.; Evstigneev, M. P.; Potebnya, G. P.; Panchuk, R. R.; Stoika, R. S.; Ritter, U.; Scharff, P. Complex of C60 fullerene with doxorubicin as a promising agent in antitumor therapy. Nanoscale Res. Lett. 2015, 10, 499.
Prylutskyy, Y. I.; Evstigneev, M. P.; Cherepanov, V. V.; Kyzyma, O. A.; Bulavin, L. A.; Davidenko, N. A.; Scharff, P. Structural organization of C60 fullerene, doxorubicin, and their complex in physiological solution as promising antitumor agents. J. Nanopart. Res. 2015, 17, 45.
Prylutskyy, Y. I.; Cherepanov, V. V.; Evstigneev, M. P.; Kyzyma, O. A.; Petrenko, V. I.; Styopkin, V. I.; Bulavin, L. A.; Davidenko, N. A.; Wyrzykowski, D.; Woziwodzka, A. et al. Structural self-organization of C60 and cisplatin in physiological solution. Phys. Chem. Chem. Phys. 2015, 17, 26084–26092.
Levi, J. A.; Aroney, R. S.; Dalley, D. N. Haemolytic anaemia after cisplatin treatment. BMJ 1981, 282, 2003–2004.
Aguilar-Markulis, N. V.; Beckley, S.; Priore, R.; Mettlin, C. Auditory toxicity effects of long-term cisdichlorodiammineplatinum II therapy in genitourinary cancer patients. J. Surg. Oncol. 1981, 16, 111–123.
Zhou, W. J.; Kavelaars, A.; Heijnen, C. J. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One 2016, 11, e0151890.
Perobelli, J. E. Effects of anticancer drugs in reproductive parameters of juvenile male animals and role of protective agents. Anticancer Agents Med. Chem., in press, DOI: 10.2174/1871520616666160219162033.
Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Crystallogr. 1977, 10, 415–421.
Glatter, O. The interpretation of real-space information from small-angle scattering experiments. J. Appl. Crystallogr. 1979, 12, 166–175.
Gao, J.; Wang, T.; Qiu, S.; Zhu, Y.; Liang, L.; Zheng, Y. Structure-based drug design of small molecule peptide deformylase inhibitors to treat cancer. Molecules 2016, 21, 396.
Fukunishi, Y.; Mashimo, T.; Misoo, K.; Wakabayashi, Y.; Miyaki, T.; Ohta, S.; Nakamura, M.; Ikeda, K. Miscellaneous topics in computer-aided drug design: Synthetic accessibility and GPU computing, and other topics. Curr. Pharm. Des. 2016, 22, 3555–3568.
Pandey, R. K.; Kumbhar, B. V.; Sundar, S.; Kunwar, A.; Prajapati, V. K. Structure-based virtual screening, molecular docking, ADMET and molecular simulations to develop benzoxaborole analogs as potential inhibitor against Leishmania donovani trypanothione reductase. J. Recept. Signal Transduct. Res., in press, DOI: 10.3109/10799893.2016.1171344.
Andreichenko, K. S.; Prylutska, S. V.; Medynska, K. O.; Bogutska, K. I.; Nurishchenko, N. E.; Prylutskyy, Y. I.; Ritter, U.; Scharff, P. Effect of fullerene C60 on ATPase activity and superprecipitation of skeletal muscle actomyosin. Ukr. Biochim. Zh. 2013, 85, 20–26.
Xu, X.; Li, R. B.; Ma, M.; Wang, X.; Wang, Y. H.; Zou, H. F. Multidrug resistance protein P-glycoprotein does not recognize nanoparticle C60: Experiment and modeling. Soft Matter 2012, 8, 2915–2923.
Liu, X. Y.; Liu, S. P.; Jiang, J.; Zhang, X.; Zhang, T. Inhibition of the JNK signaling pathway increases sensitivity of hepatocellular carcinoma cells to cisplatin by downregulating expression of P-glycoprotein. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1098–1108.
Pastan, I.; Gottesman, M. M.; Ueda, K.; Lovelace, E.; Rutherford, A. V.; Willingham, M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490.
Leslie, E. M.; Deeley, R. G.; Cole, S. P. C. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237.
Sreenivasan, S.; Ravichandran, S.; Vetrivel, U.; Krishnakumar, S. In vitro and In silico studies on inhibitory effects of curcumin on multi drug resistance associated protein (MRP1) in retinoblastoma cells. Bioinformation 2012, 8, 13–19.
Li, Q. C.; Liang, Y.; Hu, G. R.; Tian, Y. Enhanced therapeutic efficacy and amelioration of cisplatin-induced nephrotoxicity by quercetin in 1,2-dimethyl hydrazine-induced colon cancer in rats. Indian J. Pharmacol. 2016, 48, 168–171.
Kovach, J. S.; Moertel, C. G.; Schutt, A. J.; Reitemeier, R. G.; Hahn, R. G. Phase II study of cis-diamminedichloroplatinum (NSC-119875) in advanced carcinoma of the large bowel. Cancer Chemother. Rep. 1973, 57, 357–359.
Friedlander, M.; Kaye, S. B.; Sullivan, A.; Atkinson, K.; Elliott, P.; Coppleson, M.; Houghton, R.; Solomon, J.; Green, D.; Russell, P. et al. Cervical-carcinoma: A drug-responsive tumor—experience with combined cisplatin, vinblastine, and bleomycin therapy. Gynecol. Oncol. 1983, 16, 275–281.
Prestayko, A. W.; D’Aoust, J. C.; Issell, B. F.; Crooke, S. T. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat. Rev. 1979, 6, 17–39.
Hashmi, H.; Maqbool, A.; Ahmed, S.; Ahmed, A.; Sheikh, K.; Ahmed, A. Concurrent cisplatin-based chemoradiation in squamous cell carcinoma of cervix. J. Coll. Physicians Surg. Pak. 2016, 26, 302–305.
Jendželovská, Z.; Jendželovský, R.; Hilovská, L.; Koval, J.; Mikeš, J.; Fedorocko, P. Single pre-treatment with hypericin, a St. John’s wort secondary metabolite, attenuates cisplatin-and mitoxantrone-induced cell death in A2780, A2780cis and HL-60 cells. Toxicol. in Vitro 2014, 28, 1259–1273.
Xu, H.-W.; Xu, L.; Hao, J.-H.; Qin, C.-Y.; Liu, H. Expression of P-glycoprotein and multidrug resistanceassociated protein is associated with multidrug resistance in gastric cancer. J. Int. Med. Res. 2010, 38, 34–42.
Lin, X. J.; Howell, S. B. DNA mismatch repair and p53 function are major determinants of the rate of development of cisplatin resistance. Mol. Cancer Ther. 2006, 5, 1239–1247.
Sui, X.; Luo, C.; Wang, C.; Zhang, F. W.; Zhang, J. Y.; Guo, S. W. Graphene quantum dots enhance anticancer activity of cisplatin via increasing its cellular and nuclear uptake. Nanomedicine 2016, 12, 1997–2006.
He, G. D.; He, G. L.; Zhou, R. Y.; Pi, Z. B.; Zhu, T. Q.; Jiang, L. M.; Xie, Y. B. Enhancement of cisplatin induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016, 469, 1075–1082.
Desbois, N.; Pertuit, D.; Moretto, J.; Cachia, C.; Chauffert, B.; Bouyer, F. cis-Dichloroplatinum(II) complexes tethered to dibenzo[c,h][1,6]_naphthyridin-6-ones: Synthesis and cytotoxicity in human cancer cell lines in vitro. Eur. J. Med. Chem. 2013, 69, 719–727.
Lee, Y.; Kim, Y. J.; Choi, Y. J.; Lee, J. W.; Lee, S.; Chung, H. W. Enhancement of cisplatin cytotoxicity by benzyl isothiocyanate in HL-60 cells. Food Chem. Toxicol. 2012, 50, 2397–2406.
Roy, M.; Mukherjee, S. Reversal of resistance towards cisplatin by curcumin in cervical cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 1403–1410.
Neumann, W.; Crews, B. C.; Sárosi, M. B.; Daniel, C. M.; Ghebreselasie, K.; Scholz, M. S.; Marnett, L. J.; Hey-Hawkins, E. Conjugation of cisplatin analogues and cyclooxygenase inhibitors to overcome cisplatin resistance. ChemMedChem 2015, 10, 183–192.
Wang, T. H.; Wan, J. Y.; Gong, X.; Li, H. Z.; Cheng, Y. Tetrandrine enhances cytotoxicity of cisplatin in human drugresistant esophageal squamous carcinoma cells by inhibition of multidrug resistance-associated protein 1. Oncol. Rep. 2012, 28, 1681–1686.
Pariente, R.; Pariente, J. A.; Rodríguez, A. B.; Espino, J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: Effects on oxidative stress and DNA fragmentation. J. Pineal Res. 2016, 60, 55–64.
Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883.
Ormerod, M. G.; O’Neill, C. F.; Robertson, D.; Harrap, K. R. Cisplatin induces apoptosis in a human ovarian carcinoma cell line without concomitant internucleosomal degradation of DNA. Exp. Cell Res. 1994, 211, 231–237.
Ma, S. H.; Tan, W. H.; Du, B. T.; Liu, W.; Li, W. J.; Che, D. H.; Zhang, G. M. Oridonin effectively reverses cisplatin drug resistance in human ovarian cancer cells via induction of cell apoptosis and inhibition of matrix metalloproteinase expression. Mol. Med. Rep. 2016, 13, 3342–3348.
Golunski, G.; Woziwodzka, A.; Iermak, I.; Rychlowski, M.; Piosik, J. Modulation of acridine mutagen ICR191 intercalation to DNA by methylxanthines-analysis with mathematical models. Bioorg. Med. Chem. 2013, 21, 3280–3289.
Woziwodzka, A.; Gwizdek-Wisniewska, A.; Piosik, J. Caffeine, pentoxifylline and theophylline form stacking complexes with IQ-type heterocyclic aromatic amines. Bioorg. Chem. 2011, 39, 10–17.
Woziwodzka, A.; Golunski, G.; Wyrzykowski, D.; Kazmierkiewicz, R.; Piosik, J. Caffeine and other methylxanthines as interceptors of food-borne aromatic mutagens: Inhibition of Trp-P-1 and Trp-P-2 mutagenic activity. Chem. Res. Toxicol. 2013, 26, 1660–1673.
Golunski, G.; Borowik, A.; Derewonko, N.; Kawiak, A.; Rychlowski, M.; Woziwodzka, A.; Piosik, J. Pentoxifylline as a modulator of anticancer drug doxorubicin. Part II: Reduction of doxorubicin DNA binding and alleviation of its biological effects. Biochimie 2016, 123, 95–102.
Golunski, G.; Borowik, A.; Lipinska, A.; Romanik, M.; Derewonko, N.; Woziwodzka, A.; Piosik, J. Pentoxifylline affects idarubicin binding to DNA. Bioorg. Chem. 2016, 65, 118–125.
Orel, V.; Shevchenko, A.; Romanov, A.; Tselepi, M.; Mitrelias, T.; Barnes, C. H. W.; Burlaka, A.; Lukin, S.; Shchepotin, I. Magnetic properties and antitumor effect of nanocomplexes of iron oxide and doxorubicin. Nanomedicine 2015, 11, 47–55.
Prylutska, S. V.; Korolovych, V. F.; Prylutskyy, Y. I.; Evstigneev, M. P.; Ritter, U.; Scharff, P. Tumor-inhibitory effect of C60 fullerene complex with doxorubicin. Nanomed. Nanobiol. 2015, 2, 49–53.
Liu, X. X.; Liu, Y.; Hao, J. J.; Zhao, X. L.; Lang, Y. Z.; Fan, F.; Cai, C.; Li, G. Y.; Zhang, L. J.; Yu, G. L. In vivo anti-cancer mechanism of low-molecular-weight fucosylated chondroitin sulfate (LFCS) from sea cucumber Cucumaria frondosa. Molecules 2016, 21, 625.
Xu, Y. Z.; Li, Y. H.; Lu, W. J.; Lu, K.; Wang, C. T.; Li, Y.; Lin, H. J.; Kan, L. X.; Yang, S. Y.; Wang, S. Y. et al. YL4073 is a potent autophagy-stimulating antitumor agent in an in vivo model of Lewis lung carcinoma. Oncol. Rep. 2016, 35, 2081–2088.
Peng, X. C.; Chen, X. X.; Zhang, Y.; Wang, H. J.; Feng, Y. A novel inhibitor of Rho GDP-dissociation inhibitor a improves the therapeutic efficacy of paclitaxel in Lewis lung carcinoma. Biomed. Rep. 2015, 3, 473–477.
Fan, S. J.; Xu, Y.; Li, X.; Tie, L.; Pan, Y.; Li, X. J. Opposite angiogenic outcome of curcumin against ischemia and Lewis lung cancer models: In silico, in vitro and in vivo studies. Biochim. Biophys. Acta 2014, 1842, 1742–1754.
Niu, P. G.; Zhang, Y. X.; Shi, D. H.; Liu, Y.; Chen, Y. Y.; Deng, J. Cardamonin inhibits metastasis of Lewis lung carcinoma cells by decreasing mTOR activity. PLoS One 2015, 10, e0127778.
Liu, Y. Z.; Yang, C. M.; Chen, J. Y.; Liao, J. W.; Hu, M. L. Alpha-carotene inhibits metastasis in Lewis lung carcinoma in vitro, and suppresses lung metastasis and tumor growth in combination with taxol in tumor xenografted C57BL/6 mice. J. Nutr. Biochem. 2015, 26, 607–615.
Das, S. K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K. P.; Kumari, P.; Trauner, M. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238.
Tsoli, M.; Robertson, G. Cancer cachexia: Malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol. Metab. 2013, 24, 174–183.
Porporato, P. E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200.
Perego, P.; Righetti, S. C.; Supino, R.; Delia, D.; Caserini, C.; Carenini, N.; Bedogné, B.; Broome, E.; Krajewski, S.; Reed, J. C. et al. Role of apoptosis and apoptosis-related proteins in the cisplatin-resistant phenotype of human tumor cell lines. Apoptosis 1997, 2, 540–548.
Jinushi, M. Immune regulation of therapy-resistant niches: Emerging targets for improving anticancer drug responses. Cancer Metastasis Rev. 2014, 33, 737–745.
D’Arena, G.; Deaglio, S.; Laurenti, L.; de Martino, L.; de Feo, V.; Fusco, B. M.; Carella, A. M.; Cascavilla, N.; Musto, P. Targeting regulatory T cells for anticancer therapy. Mini Rev. Med. Chem. 2011, 11, 480–485.
Shurin, M. R.; Naiditch, H.; Gutkin, D. W.; Umansky, V.; Shurin, G. V. ChemoImmunoModulation: Immune regulation by the antineoplastic chemotherapeutic agents. Curr. Med. Chem. 2012, 19, 1792–1803.
Skivka, L. M.; Fedorchuk, O. G.; Bezdeneznykh, N. O.; Lykhova, O. O.; Semesiuk, N. I.; Kudryavets, Y. I.; Malanchuk, O. M. The effect of antineoplastic drug NSC63150 on immunogenicity of B16 melanoma. J. Exp. Integr. Med. 2014, 4, 93–105.
Fedorchuk, O. G.; Pyaskovskaya, O. M.; Skivka, L. M.; Gorbik, G. V.; Trompak, O. O.; Solyanik, G. I. Paraneoplastic syndrome in mice bearing high-angiogenic variant of Lewis lung carcinoma: Relations with tumor derived VEGF. Cytokine 2012, 57, 81–88.
Yang, X. L.; Ebrahimi, A.; Li, J.; Cui, Q. J. Fullerenebiomolecule conjugates and their biomedicinal applications. Int. J. Nanomedicine 2014, 9, 77–92.
Turabekova, M.; Rasulev, B.; Theodore, M.; Jackman, J.; Leszczynska, D.; Leszczynski, J. Immunotoxicity of nanoparticles: A computational study suggests that CNTs and C60 fullerenes might be recognized as pathogens by Toll-like receptors. Nanoscale 2014, 6, 3488–3495.
Prylutska, S. V.; Grynyuk, I. I.; Grebinyk, S. M.; Matyshevska, O. P.; Prylutskyy, Y. I.; Ritter, U.; Siegmund, C.; Scharff, P. Comperative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materialwiss. Werkstofftech. 2009, 40, 238–241.
Prylutskyy, Y. I.; Petrenko, V. I.; Ivankov, O. I.; Kyzyma, O. A.; Bulavin, L. A.; Litsis, O. O.; Evstigneev, M. P.; Cherepanov, V. V.; Naumovets, A. G.; Ritter, U. On the origin of C60 fullerene solubility in aqueous solution. Langmuir 2014, 30, 3967–3970.
Ritter, U.; Prylutskyy, Y. I.; Evstigneev, M. P.; Davidenko, N. A.; Cherepanov, V. V.; Senenko, A. I.; Marchenko, O. A.; Naumovets, A. G. Structural features of highly stable reproducible C60 fullerene aqueous colloid solution probed by various techniques. Fullerenes, Nanotubes, Carbon Nanostruct. 2015, 23, 530–534.
Blanton, T. N.; Barnes, C. L.; Lelental, M. Preparation of silver behenate coatings to provide low-to mid-angle diffraction calibration. J. Appl. Cryst. 2000, 33, 172–173.
Franke, D.; Kikhney, A. G.; Svergun, D. I. Automated acquisition and analysis of small angle X-ray scattering data. Nucl. Inst. Meth. Phys. Res. Sect. A 2012, 689, 52–59.
Svergun, D. I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Cryst. 1992, 25, 495–503.
Li, J. Z.; Jaimes, K. F.; Aller, S. G. Refined structures of mouse P-glycoprotein. Protein Sci. 2014, 23, 34–46.
Ramaen, O.; Leulliot, N.; Sizun, C.; Ulryck, N.; Pamlard, O.; Lallemand, J. Y.; van Tilbeurgh, H.; Jacquet, E. Structure of the human multidrug resistance protein 1 nucleotide binding domain 1 bound to Mg2+/ATP reveals a non-productive catalytic site. J. Mol. Biol. 2006, 359, 940–949.
Vedadi, M.; Lew, J.; Artz, J.; Amani, M.; Zhao, Y.; Dong, A. P.; Wasney, G. A.; Gao, M.; Hills, T.; Brokx, S. et al. Genome-scale protein expression ans structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol. Biochem. Parasit. 2007, 151, 100–110.
Warren, G. L.; Andrews, C. W.; Capelli, A. M.; Clarke, B.; LaLonde, J.; Lambert, M. H.; Lindvall, M.; Nevins, N.; Semus, S. F.; Senger, S. et al. A critical assessment of docking programs and scoring functions. J. Med. Chem. 2006, 49, 5912–5931.
McMartin, C.; Bohacek, R. S. QXP: Powerful, rapid computer algorithms for structure-based drug design. J. Comput. Aided Mol. Des. 1997, 11, 333–344.
Walker, P. R.; Kwast-Welfeld, J.; Gourdeau, H.; Leblanc, J.; Neugebauer, W.; Sikorska, M. Relationship between apoptosis and the cell cycle in lymphocytes: Roles of protein kinase C, tyrosine phosphorylation, and AP1. Exp. Cell Res. 1993, 207, 142–151.
Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60.
Acknowledgements
We gratefully acknowledge the technical support from Clement Blanchet (EMBL) at the P12 BioSAXS beamline (EMBL/DESY, PETRA III). The research was partially supported by Russian Science Fund (No. 14-14-00328). S. Prylutska receives financial support by the Academician Platon Kostyuk Foundation, R. Panchuk receives financial support by West-Ukrainian BioMedical Research Center (WUMBRC) and by grant of Nationl Academy of Sciences of Ukraine for young scientists.
Author information
Authors and Affiliations
Corresponding authors
Additional information
These authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Prylutska, S., Panchuk, R., Gołuński, G. et al. C60 fullerene enhances cisplatin anticancer activity and overcomes tumor cell drug resistance. Nano Res. 10, 652–671 (2017). https://doi.org/10.1007/s12274-016-1324-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1324-2