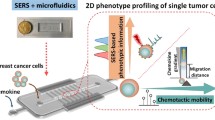
Abstract
Discovering novel drugs for cancer immunotherapy requires a robust in vitro drug screening platform that allows for straightforward probing of cell–cell communications. Here, we combined surface-enhanced Raman scattering (SERS) nanoprobes with microfluidic networks to monitor in situ the cancer–immune system intercellular communications. The microfluidic platform links up immune cells with cancer cells, where the cancer-cell secretions act as signaling mediators. First, gold@silver core–shell nanorods were employed to fabricate SERS immunoprobes for analysis of the signaling molecules. Multiple cancer secretions in a tumor microenvironment were quantitatively analyzed by a SERS-assisted three-dimensional (3D) barcode immunoassay with high sensitivity (1 ng/mL). Second, in an on-chip cell proliferation assay, multiple immunosuppressive proteins secreted by cancer cells were found to inhibit activation of immune cells, indicating that the platform simulates the physiological process of cancer–immune system communications. Furthermore, potential drug candidates were tested on this platform. A quantitative SERS immunoassay was performed to evaluate drug efficacy at regulating the secretion behavior of cancer cells and the activity of immune cells. This assay showed the suitability of this platform for in vitro drug screening. It is expected that the fully integrated and highly automated SERS-microfluidic platform will become a powerful analytical tool for probing intercellular communications and should accelerate the discovery and clinical validation of novel drugs.

Similar content being viewed by others
References
Van der Burg, S. H.; Arens, R.; Ossendorp, F.; van Hall, T.; Melief, C. J. M. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 2016, 16, 219–233.
Kassiotis, G.; Stoye, J. P. Immune responses to endogenous retroelements: Taking the bad with the good. Nat. Rev. Immunol. 2016, 16, 207–219.
Obenauf, A. C.; Zou, Y. L.; Ji, A. L.; Vanharanta, S.; Shu, W. P.; Shi, H. B.; Kong, X. J.; Bosenberg, M. C.; Wiesner, T.; Rosen, N. et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015, 520, 368–372.
Motz, G. T.; Coukos, G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat. Rev. Immunol. 2011, 11, 702–711.
Vinay, D. S.; Ryan, E. P.; Pawelec, G.; Talib, W. H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W. K.; Whelan, R. L.; Kumara, H. et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198.
Kim, R.; Emi, M.; Tanabe, K.; Arihiro, K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006, 66, 5527–5536.
Venuti, A.; Curzio, G.; Mariani, L.; Paolini, F. Immunotherapy of HPV-associated cancer: DNA/plant-derived vaccines and new orthotopic mouse models. Cancer Immunol. Immunother. 2015, 64, 1329–1338.
Ostrand-Rosenberg, S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr. Opin. Immunol. 2004, 16, 143–150.
Imhof, M.; Karas, I.; Gomez, I.; Eger, A.; Imhof, M. Interaction of tumor cells with the immune system: Implications for dendritic cell therapy and cancer progression. Drug Discov. Today 2013, 18, 35–42.
López-Muñoz, H.; Escobar-Sánchez, M. L.; López-Marure, R.; Lascurain-Ledesma, R.; Zenteno, E.; Hernández-Vazquez, J. M. V.; Weißs-Steider, B.; Sánchez-Sánchez, L. Cervical cancer cells induce apoptosis in TCD4+lymphocytes through the secretion of TGF-ß. Arch. Gynecol. Obstet. 2013, 287, 755–763.
Joffroy, C. M.; Buck, M. B.; Stope, M. B.; Popp, S. L.; Pfizenmaier, K.; Knabbe, C. Antiestrogens induce transforming growth factor ß-mediated immunosuppression in breast cancer. Cancer Res. 2010, 70, 1314–1322.
Díaz-Benítez, C. E.; Navarro-Fuentes, K. R.; Flores-Sosa, J. A.; Juárez-Díaz, J.; Uribe-Salas, F. J.; Román-Basaure, E.; González-Mena, L. E.; Alonso de Ruíz, P.; López-Estrada, G.; Lagunas-Martínez, A. et al. CD3? expreßsion and T cell proliferation are inhibited by TGF-ß1 and IL-10 in cervical cancer patients. J. Clin. Immunol. 2009, 29, 532–544.
Garnett, M. J.; Edelman, E. J.; Heidorn, S. J.; Greenman, C. D.; Dastur, A.; Lau, K. W.; Greninger, P.; Thompson, I. R.; Luo, X.; Soares, J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012, 483, 570–575.
Gao, H.; Korn, J. M.; Ferretti, S.; Monahan, J. E.; Wang, Y. Z.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G. Z.; Zhang, Y. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015, 21, 1318–1325.
Neužil, P.; Giselbrecht, S.; Länge, K.; Huang, T. J.; Manz, A. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632.
Zheng, X. T.; Yu, L.; Li, P. W.; Dong, H.; Wang, Y. J.; Liu, Y.; Li, C. M. On-chip investigation of cell-drug interactions. Adv. Drug Deliv. Rev. 2013, 65, 1556–1574.
Shao, Y.; Fu, J. P. Integrated micro/nanoengineered functional biomaterials for cell mechanics and mechanobiology: A materials perspective. Adv. Mater. 2014, 26, 1494–1533.
Kim, D.; Wu, X. J.; Young, A. T.; Haynes, C. L. Microfluidics-based in vivo mimetic systems for the study of cellular biology. Acc. Chem. Res. 2014, 47, 1165–1173.
Nahavandi, S.; Tang, S. Y.; Baratchi, S.; Soffe, R.; Nahavandi, S.; Kalantar-Zadeh, K.; Mitchell, A.; Khoshmanesh, K. Microfluidic platforms for the investigation of intercellular signalling mechanisms. Small 2014, 10, 4810–4826.
Guo, F.; French, J. B.; Li, P.; Zhao, H.; Chan, C. Y.; Fick, J. R.; Benkovic, S. J.; Huang, T. J. Probing cell-cell communication with microfluidic devices. Lab Chip 2013, 13, 3152–3162.
Unger, M. A.; Chou, H. P.; Thorsen, T.; Scherer, A.; Quake, S. R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116.
Zeng, S. J.; Li, B. W.; Su, X. O.; Qin, J. H.; Lin, B. C. Microvalve-actuated precise control of individual droplets in microfluidic devices. Lab Chip 2009, 9, 1340–1343.
Liu, J.; Hansen, C.; Quake, S. R. Solving the “world-to-chip” interface problem with a microfluidic matrix. Anal. Chem. 2003, 75, 4718–4723.
Lane, L. A.; Qian, X. M.; Nie, S. M. SERS nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529.
Wang, Y. Q.; Yan, B.; Chen, L. X. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428.
Wu, L.; Wang, Z. Y.; Fan, K. Q.; Zong, S. F.; Cui, Y. P. A SERS-assisted 3D barcode chip for high-throughput biosensing. Small 2015, 11, 2798–2806.
Dogar, A. M.; Towbin, H.; Hall, J. Suppreßsion of latent transforming growth factor (TGF)-ß1 restores growth inhibitory TGF-ß signaling through microRNAs. J. Biol. Chem. 2011, 286, 16447–16458.
Chou, S.-Y.; Hsu, C.-S.; Hsu, M.-Y.; Liang, S.-J.; Yeh, C.-L.; Yeh, S.-L. Effects of different arginine concentrations on angiogenic protein production induced by HeLa cells. Nutrition 2010, 26, 818–822.
Vardhan, H.; Gupta, R.; Jha, R.; Bhengraj, A. R.; Mittal, A. Ferritin heavy chain-mediated iron homoeostasis regulates expression of IL-10 in Chlamydia trachomatis-infected HeLa cells. Cell Biol. Int. 2011, 35, 793–798.
Cui, C.; Feng, H. L.; Shi, X. L.; Wang, Y. Z.; Feng, Z. Y.; Liu, J. L.; Han, Z. P.; Fu, J. Q.; Fu, Z. J.; Tong, H. Artesunate down-regulates immunosuppreßsion from colorectal cancer Colon26 and RKO cells in vitro by decreasing transforming growth factor ß1 and interleukin-10. Int. Immunopharmacol. 2015, 27, 110–121.
Zuo, W.; Wang, Z. Z.; Xue, J. Artesunate induces apoptosis of bladder cancer cells by miR-16 regulation of COX-2 expression. Int. J. Mol. Sci. 2014, 15, 14298–14312.
Michaelis, M.; Kleinschmidt, M. C.; Barth, S.; Rothweiler, F.; Geiler, J.; Breitling, R.; Mayer, B.; Deubzer, H.; Witte, O.; Kreuter, J. et al. Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem. Pharmacol. 2010, 79, 130–136.
Youns, M.; Efferth, T.; Reichling, J.; Fellenberg, K.; Bauer, A.; Hoheisel, J. D. Gene expression profiling identifies novel key players involved in the cytotoxic effect of Artesunate on pancreatic cancer cells. Biochem. Pharmacol. 2009, 78, 273–283.
Sideras, K.; Braat, H.; Kwekkeboom, J.; van Eijck, C. H.; Peppelenbosch, M. P.; Sleijfer, S.; Bruno, M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat. Rev. 2014, 40, 513–522.
Leen, A. M.; Rooney, C. M.; Foster, A. E. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007, 25, 243–265.
Zhang, H.; Song, Y.; Li, Z. Y.; Zhang, T.; Zeng, L.; Li, W. L.; Bian, Y. Y. Evaluation of ligustrazine on the prevention of experimentally induced abdominal adhesions in rats. Int. J. Surg. 2015, 21, 115–121.
Wu, L.; Wang, Z. Y.; Zong, S. F.; Huang, Z.; Zhang, P. Y.; Cui, Y. P. A SERS-based immunoassay with highly increased sensitivity using gold/silver core-shell nanorods. Biosens. Bioelectron. 2012, 38, 94–99.
Acknowledgements
This research was supported by the National Natural Science of China (No. 61535003, 61177033 and 61275182), the Excellent Youth Foundation of Jiangsu Province (No. BK20140023), the National Basic Research Program of China (No. 2015CB352002), the Scientific Research Foundation of Graduate School of Southeast University (No. YBPY1507), the Science Foundation for the Excellent Youth Scholars of Southeast University and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2016_1316_MOESM1_ESM.pdf
In situ probing of cell–cell communications with surfaceenhanced Raman scattering (SERS) nanoprobes and microfluidic networks for screening of immunotherapeutic drugs
Rights and permissions
About this article
Cite this article
Wu, L., Wang, Z., Zhang, Y. et al. In situ probing of cell–cell communications with surface-enhanced Raman scattering (SERS) nanoprobes and microfluidic networks for screening of immunotherapeutic drugs. Nano Res. 10, 584–594 (2017). https://doi.org/10.1007/s12274-016-1316-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1316-2