Abstract
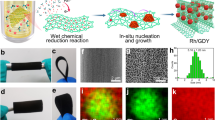
Nanocrystals of Rh, an important member of the noble metal catalyst family, have wide applications in heterogeneous catalytic reactions. Controlling the morphology of these noble metal nanocrystals has become an effective strategy for improving their catalytic activity and durability. In this work, well-defined Rh nanodendrites with very thin triangular branches as subunits are synthesized using a facile diethylene glycol reduction method, assisted by polyethyleneimine as a complex-forming agent and surfactant. For the first time, the methanol oxidation reaction (MOR) on Rh nanocrystals with a well-defined morphology is investigated using various electrochemical techniques in an alkaline medium. Unexpectedly, the as-prepared Rh nanodendrites, with ultrathin nanosheet subunits, exhibit superior electrocatalytic activity and durability during the MOR in an alkaline medium, indicating that Rh nanocrystals with specific morphology may be highly promising alternatives to Pt electrocatalysts in the MOR in an alkaline medium.

Similar content being viewed by others
References
Bianchini, C.; Shen, P. K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206.
Antolini, E.; Gonzalez, E. R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450.
Lu, Y. Z.; Jiang, Y. Y.; Gao, X. H.; Wang, X. D.; Chen, W. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697.
Shen, M. X.; Zheng, L.-R.; He, W. H.; Ruan, C. P.; Jiang, C. H.; Ai, K. L.; Lu, L. H. High-performance oxygen reduction electrocatalysts derived from uniform cobalt–adenine assemblies. Nano Energy 2015, 17, 120–130.
Huang, W. J.; Wang, H. T.; Zhou, J. G.; Wang, J.; Duchesne, P. N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F. P.; Zeng, M. et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015, 6, 10035.
Koenigsmann, C.; Wong, S. S. Tailoring chemical composition to achieve enhanced methanol oxidation reaction and methanol-tolerant oxygen reduction reaction performance in palladium-based nanowire systems. ACS Catal. 2013, 3, 2031–2040.
Kannan, P.; Maiyalagan, T.; Opallo, M. One-pot synthesis of chain-like palladium nanocubes and their enhanced electrocatalytic activity for fuel-cell applications. Nano Energy 2013, 2, 677–687.
Wang, N.; Xu, Y.; Han, Y.; Gao, C. Z.; Cao, X. Mesoporous Pd@M (M = Pt, Au) microrods as excellent electrocatalysts for methanol oxidation. Nano Energy 2015, 17, 111–119.
Zhao, M.; Abe, K.; Yamaura, S.-I.; Yamamoto, Y.; Asao, N. Fabrication of Pd–Ni–P metallic glass nanoparticles and their application as highly durable catalysts in methanol electro-oxidation. Chem. Mater. 2014, 26, 1056–1061.
Xu, L.; Luo, Z. M.; Fan, Z. X.; Yu, S. J.; Chen, J. Z.; Liao, Y. S.; Xue, C. Controllable galvanic synthesis of triangular Ag–Pd alloy nanoframes for efficient electrocatalytic methanol oxidation. Chem.—Eur. J. 2015, 21, 8691–8695.
Shen, S. Y.; Zhao, T. S. One-step polyol synthesis of Rhon- Pd bimetallic nanodendrites and their electrocatalytic properties for ethanol oxidation in alkaline media. J. Mater. Chem. A 2013, 1, 906–912.
Suo, Y.; Hsing, I.-M. Highly active rhodium/carbon nanocatalysts for ethanol oxidation in alkaline medium. J. Power Sources 2011, 196, 7945–7950.
Zhang, F. F.; Zhou, D. B.; Zhang, Z. J.; Zhou, M. D.; Wang, Q. Preparation of Rh/C and its high electro-catalytic activity for ethanol oxidation in alkaline media. RSC Adv. 2015, 5, 91829–91835.
Yuan, Q.; Zhou, Z. Y.; Zhuang, J.; Wang, X. Tunable aqueous phase synthesis and shape-dependent electrochemical properties of rhodium nanostructures. Inorg. Chem. 2010, 49, 5515–5521.
Sathe, B. R. Methanol electro-oxidation on nanostructured rhodium network. Energy Environ. Focus 2015, 4, 196–200.
Wu, Z. X.; Chen, W. L.; Liu, H. Y.; Zhai, P.; Xiao, C. X.; Su, D. S.; Liu, H. C.; Ma, D. Reconstruction of rh nanoparticles in methanol oxidation reaction. Catal. Sci. Technol. 2015, 5, 4116–4122.
Ye, E. Y.; Regulacio, M. D.; Zhang, S.-Y.; Loh, X. J.; Han, M.-Y. Anisotropically branched metal nanostructures. Chem. Soc. Rev. 2015, 44, 6001–6017.
Xie, S. F.; Liu, X. Y.; Xia, Y. N. Shape-controlled syntheses of rhodium nanocrystals for the enhancement of their catalytic properties. Nano Res. 2015, 8, 82–96.
Porter, N. S.; Wu, H.; Quan, Z. W.; Fang, J. Y. Shape-control and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877.
Chen, A. C.; Ostrom, C. Palladium-based nanomaterials: Synthesis and electrochemical applications. Chem. Rev. 2015, 115, 11999–12044.
Zhang, L. L.; Wei, M.; Wang, S. Q.; Li, Z.; Ding, L.-X.; Wang, H. H. Highly stable PtP alloy nanotube arrays as a catalyst for the oxygen reduction reaction in acidic medium. Chem. Sci. 2015, 6, 3211–3216.
Xu, H.; Ding, L.-X.; Feng, J.-X.; Li, G.-R. Pt/Ni(OH)2-NiOOH/Pd multi-walled hollow nanorod arrays as superior electrocatalysts for formic acid electrooxidation. Chem. Sci. 2015, 6, 6991–6998.
Long, R.; Zhou, S.; Wiley, B. J.; Xiong, Y. J. Oxidative etching for controlled synthesis of metal nanocrystals: Atomic addition and subtraction. Chem. Soc. Rev. 2014, 43, 6288–6310.
Wang, A.-L.; Liang, C.-L.; Lu, X.-F.; Tong, Y.-X.; Li, G.-R. Pt-MoO3-RGO ternary hybrid hollow nanorod arrays as high-performance catalysts for methanol electrooxidation. J. Mater. Chem. A 2016, 4, 1923–1930.
Ding, L.-X.; Wang, A.-L.; Li, G.-R.; Liu, Z.-Q.; Zhao, W.-X.; Su, C.-Y.; Tong, Y.-X. Porous Pt-Ni-P composite nanotube arrays: Highly electroactive and durable catalysts for methanol electrooxidation. J. Am. Chem. Soc. 2012, 134, 5730–5733.
Ma, L.; Wang, C. M.; Xia, B. Y.; Mao, K. K.; He, J. W.; Wu, X. J.; Xiong, Y. J.; Lou, X. W. Platinum multicubes prepared by Ni2+-mediated shape evolution exhibit high electrocatalytic activity for oxygen reduction. Angew. Chem., Int. Ed. 2015, 54, 5666–5671.
Cheong, W.-C.; Liu, C. H.; Jiang, M. L.; Duan, H. H.; Wang, D. S.; Chen, C.; Li, Y. D. Free-standing palladiumnickel alloy wavy nanosheets. Nano Res. 2016, 9, 2244–2250.
Liu, D.; Xie, M. L.; Wang, C. M.; Liao, L. W.; Qiu, L.; Ma, J.; Huang, H.; Long, R.; Jiang, J.; Xiong, Y. J. Pd-Ag alloy hollow nanostructures with interatomic charge polarization for enhanced electrocatalytic formic acid oxidation. Nano Res. 2016, 9, 1590–1599.
Zhu, C. Z.; Du, D.; Eychmüller, A.; Lin, Y. H. Engineering ordered and nonordered porous noble metal nanostructures: Synthesis, assembly, and their applications in electrochemistry. Chem. Rev. 2015, 115, 8896–8943.
Yang, T.; Ma, Y. X.; Huang, Q. L.; Cao, G. J. Palladium–iridium nanocrystals for enhancement of electrocatalytic activity toward oxygen reduction reaction. Nano Energy 2016, 19, 257–268.
Wang, L.; Yamauchi, Y. Metallic nanocages: Synthesis of bimetallic Pt–Pd hollow nanoparticles with dendritic shells by selective chemical etching. J. Am. Chem. Soc. 2013, 135, 16762–16765.
Jiang, B.; Li, C. L.; Malgras, V.; Imura, M.; Tominakaa, S.; Yamauchi, Y. Mesoporous Pt nanospheres with designed pore surface as highly active electrocatalyst. Chem. Sci. 2016, 7, 1575–1581.
Wang, L.; Nemoto, Y.; Yamauchi, Y. Direct synthesis of spatially-controlled Pt-on-Pd bimetallic nanodendrites with superior electrocatalytic activity. J. Am. Chem. Soc. 2011, 133, 9674–9677.
Lim, B.; Jiang, M. J.; Camargo, P. H. C.; Cho, E. C.; Tao, J.; Lu, X. M.; Zhu, Y. M.; Xia, Y. N. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305.
Wang, D.-Y.; Chou, H.-L.; Cheng, C.-C.; Wu, Y.-H.; Tsai, C.-M.; Lin, H.-Y.; Wang, Y.-L.; Hwang, B.-J.; Chen, C.-C. Fept nanodendrites with high-index facets as active electrocatalysts for oxygen reduction reaction. Nano Energy 2015, 11, 631–639.
Li, F.-M.; Gao, X.-Q.; Li, S.-N.; Chen, Y.; Lee, J.-M. Thermal decomposition synthesis of functionalized pdpt alloy nanodendrites with high selectivity for oxygen reduction reaction. NPG Asia Mater. 2015, 7, e219.
Gong, M. X.; Fu, G. T.; Chen, Y.; Tang, Y. W.; Lu, T. H. Autocatalysis and selective oxidative etching induced synthesis of platinum–copper bimetallic alloy nanodendrites electrocatalysts. ACS Appl. Mater. Interfaces 2014, 6, 7301–7308.
Guo, W. S.; Pleixats, R.; Shafir, A.; Parella, T. Rhodium nanoflowers stabilized by a nitrogen-rich peg-tagged substrate as recyclable catalyst for the stereoselective hydrosilylation of internal alkynes. Adv. Synth. Catal. 2015, 357, 89–99.
Yuan, Q.; Wang, X. Aqueous-based route toward noble metal nanocrystals: Morphology-controlled synthesis and their applications. Nanoscale 2010, 2, 2328–2335.
Feng, Y.; Ma, X. H.; Han, L.; Peng, Z. J.; Yang, J. A universal approach to the synthesis of nanodendrites of noble metals. Nanoscale 2014, 6, 6173–6179.
Zhang, H.; Li, W. Y.; Jin, M. S.; Zeng, J.; Yu, T.; Yang, D. R.; Xia, Y. N. Controlling the morphology of rhodium nanocrystals by manipulating the growth kinetics with a syringe pump. Nano Lett. 2011, 11, 898–903.
Zhang, H.; Xia, X. H.; Li, W. Y.; Zeng, J.; Dai, Y. Q.; Yang, D. R.; Xia, Y. N. Facile synthesis of five-fold twinned, starfish-like rhodium nanocrystals by eliminating oxidative etching with a chloride-free precursor. Angew. Chem., Int. Ed. 2010, 49, 5296–5300.
Yu, N. F.; Tian, N.; Zhou, Z. Y.; Huang, L.; Xiao, J.; Wen, Y. H.; Sun, S. G. Electrochemical synthesis of tetrahexahedral rhodium nanocrystals with extraordinarily high surface energy and high electrocatalytic activity. Angew. Chem., Int. Ed. 2014, 53, 5097–5101.
Mohamed, H. D. A.; Watson, S. M. D.; Horrocks, B. R.; Houlton, A. Chemical and electrochemical routes to DNAtemplated rhodium nanowires. J. Mater. Chem. C 2015, 3, 438–446.
Jiang, Y. Q.; Su, J. Y.; Yang, Y.; Jia, Y. Y.; Chen, Q. L.; Xie, Z. X.; Zheng, L. S. A facile surfactant-free synthesis of Rh flower-like nanostructures constructed from ultrathin nanosheets and their enhanced catalytic properties. Nano Res. 2016, 9, 849–856.
Zhao, L.; Xu, C. F.; Su, H. F.; Liang, J. H.; Lin, S. C.; Gu, L.; Wang, X. L.; Chen, M.; Zheng, N. F. Single-crystalline rhodium nanosheets with atomic thickness. Adv. Sci. 2015, 2, 1500100.
Duan, H. H.; Yan, N.; Yu, R.; Chang, C. R.; Zhou, G.; Hu, H. S.; Rong, H. P.; Niu, Z. Q.; Mao, J. J.; Asakura, H. et al. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, 3093.
Mazumder, V.; Sun, S. H. Oleylamine-mediated synthesis of Pd nanoparticles for catalytic formic acid oxidation. J. Am. Chem. Soc. 2009, 131, 4588–4589.
Fu, G. T.; Jiang, X.; Gong, M. X.; Chen, Y.; Tang, Y. W.; Lin, J.; Lu, T. H. Highly branched platinum nanolance assemblies by polyallylamine functionalization as superior active, stable, and alcohol-tolerant oxygen reduction electrocatalysts. Nanoscale 2014, 6, 8226–8234.
Lim, B.; Xia, Y. N. Metal nanocrystals with highly branched morphologies. Angew. Chem., Int. Ed. 2011, 50, 76–85.
Ortiz, N.; Skrabalak, S. E. Manipulating local ligand environments for the controlled nucleation of metal nanoparticles and their assembly into nanodendrites. Angew. Chem., Int. Ed. 2012, 51, 11757–11761.
Watt, J.; Cheong, S.; Tilley, R. D. How to control the shape of metal nanostructures in organic solution phase synthesis for plasmonics and catalysis. Nano Today 2013, 8, 198–215.
Gao, X. Q.; Li, Y. M.; Zhang, Q.; Li, S. N.; Chen, Y.; Lee, J.-M. Polyethyleneimine-assisted synthesis of high-quality platinum/graphene hybrids: The effect of molecular weight on electrochemical properties. J. Mater. Chem. A 2015, 3, 12000–12004.
Bai, J.; Fang, C. L.; Liu, Z. H.; Chen, Y. A one-pot gold seedassisted synthesis of gold/platinum wire nanoassemblies and their enhanced electrocatalytic activity for the oxidation of oxalic acid. Nanoscale 2016, 8, 2875–2880.
Xu, G.-R.; Liu, F.-Y.; Liu, Z.-H.; Chen, Y. Ethanol-tolerant polyethyleneimine functionalized palladium nanowires in alkaline media: The “molecular window gauze” induced the selectivity for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 21083–21089.
Huang, X. Q.; Tang, S. H.; Mu, X. L.; Dai, Y.; Chen, G. X.; Zhou, Z. Y.; Ruan, F. X.; Yang, Z. L.; Zheng, N. F. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32.
Jang, K.; Kim, H. J.; Son, S. U. Low-temperature synthesis of ultrathin rhodium nanoplates via molecular orbital symmetry interaction between rhodium precursors. Chem. Mater. 2010, 22, 1273–1275.
Hou, C. P.; Zhu, J.; Liu, C.; Wang, X.; Kuang, Q.; Zheng, L. S. Formaldehyde-assisted synthesis of ultrathin Rh nanosheets for applications in COoxidation. CrystEngComm 2013, 15, 6127–6130.
Qi, Y.; Wu, J. B.; Zhang, H.; Jiang, Y. Y.; Jin, C. H.; Fu, M. S.; Yang, H.; Yang, D. R. Facile synthesis of Rh–Pd alloy nanodendrites as highly active and durable electrocatalysts for oxygen reduction reaction. Nanoscale 2014, 6, 7012–7018.
Zhang, L.; Niu, W. X.; Gao, W. Y.; Qi, L. M.; Lai, J. P.; Zhao, J. M.; Xu, G. B. Synthesis of convex hexoctahedral palladium@gold core–shell nanocrystals with {431} highindex facets with remarkable electrochemiluminescence activities. ACS Nano 2014, 8, 5953–5958.
Quan, Z.; Wang, Y.; Fang, J. High-index faceted noble metal nanocrystals. Acc. Chem. Res. 2013, 46, 191–202.
Yu, T.; Kim, D. Y.; Zhang, H.; Xia, Y. N. Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction. Angew. Chem., Int. Ed. 2011, 50, 2773–2777.
Huang, X. Q.; Zhao, Z. P.; Fan, J. M.; Tan, Y. M.; Zheng, N. F. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721.
Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with highindex facets and high electro-oxidation activity. Science 2007, 316, 732–735.
Deng, Y.-J.; Tian, N.; Zhou, Z.-Y.; Huang, R.; Liu, Z.-L.; Xiao, J.; Sun, S.-G. Alloy tetrahexahedral Pd–Pt catalysts: Enhancing significantly the catalytic activity by synergy effect of high-index facets and electronic structure. Chem. Sci. 2012, 3, 1157–1161.
Xia, B. Y.; Wu, H. B.; Wang, X.; Lou, X. W. D. Highly concave platinum nanoframes with high-index facets and enhanced electrocatalytic properties. Angew. Chem., Int. Ed. 2013, 52, 12337–12340.
Xu, X. L.; Zhang, X.; Sun, H.; Yang, Y.; Dai, X. P.; Gao, J. S.; Li, X. Y.; Zhang, P. F.; Wang, H. H.; Yu, N. F. et al. Synthesis of Pt–Ni alloy nanocrystals with high-index facets and enhanced electrocatalytic properties. Angew. Chem. 2014, 126, 12730–12735.
Funatsu, A.; Tateishi, H.; Hatakeyama, K.; Fukunaga, Y.; Taniguchi, T.; Koinuma, M.; Matsuura, H.; Matsumoto, Y. Synthesis of monolayer platinum nanosheets. Chem. Commun. 2014, 50, 8503–8506.
Li, H.; Chen, G. X.; Yang, H. Y.; Wang, X. L.; Liang, J. H.; Liu, P. X.; Chen, M.; Zheng, N. F. Shape-controlled synthesis of surface-clean ultrathin palladium nanosheets by simply mixing a dinuclear PdI carbonyl chloride complex with H2O. Angew. Chem., Int. Ed. 2013, 52, 8368–8372.
Saleem, F.; Xu, B.; Ni, B.; Liu, H. L.; Nosheen, F.; Li, H. Y.; Wang, X. Atomically thick Pt-Cu nanosheets: Self-assembled sandwich and nanoring-like structures. Adv. Mater. 2015, 27, 2013–2018.
Saleem, F.; Zhang, Z. C.; Xu, B.; Xu, X. B.; He, P. L.; Wang, X. Ultrathin Pt-Cu nanosheets and nanocones. J. Am. Chem. Soc. 2013, 135, 18304–18307.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2016_1258_MOESM1_ESM.pdf
Unexpected catalytic activity of rhodium nanodendrites with nanosheet subunits for methanol electrooxidation in an alkaline medium
Rights and permissions
About this article
Cite this article
Kang, Y., Li, F., Li, S. et al. Unexpected catalytic activity of rhodium nanodendrites with nanosheet subunits for methanol electrooxidation in an alkaline medium. Nano Res. 9, 3893–3902 (2016). https://doi.org/10.1007/s12274-016-1258-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1258-8