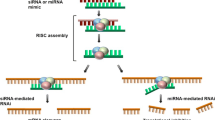
Abstract
The therapeutic promise of small interfering RNAs (siRNAs) for specific gene silencing is dependent on the successful delivery of functional siRNAs to the cytoplasm. Their conjugation to an established delivery platform, such as gold nanoparticles, offers tremendous potential for treating diseases and advancing our understanding of cellular processes. Their success or failure is dependent on both the uptake of the nanoparticles into the cells and subsequent intracellular release of the functional siRNA. In this study, utilizing gold nanoparticle siRNA-mediated delivery against C-MYC, we aimed to determine if we could achieve knockdown in a cancer cell line with low levels of intracellular glutathione, and determine the influence, if any, of polyethylene glycol (PEG) ligand density on knockdown, with a view to determining the optimal nanoparticle design to achieve C-MYC knockdown. We demonstrate that, regardless of the PEG density, knockdown in cells with relatively low glutathione levels can be achieved, as well as the possible effect of steric hindrance of PEG on the availability of the siRNA for cleavage in the intracellular environment. Gold nanoparticle uptake was demonstrated via transmission electron microscopy and mass spectroscopy, while knockdown was determined at the protein and physiological levels (cells in S-phase) by in-cell westerns and BrdU incorporation, respectively.

Similar content being viewed by others
References
Chiu, Y.-L.; Rana, T. M. siRNA function in RNAi: A chemical modification analysis. RNA 2003, 9, 1034–1048.
Derfus, A. M.; Chen, A. A.; Min, D.-H.; Ruoslahti, E.; Bhatia, S. N. Targeted quantum dot conjugates for siRNA delivery. Bioconjugate Chem. 2007, 18, 1391–1396.
Grigsby, C. L.; Leong, K. W. Balancing protection and release of DNA: Tools to address a bottleneck of non-viral gene delivery. J. R. Soc. Interface 2010, 7, S67–S82.
Lévy, R.; Shaheen, U.; Cesbron, Y.; Sée, V. Gold nanoparticles delivery in mammalian live cells: A critical review. Nano Rev. 2010, 1, 4889.
Karakoti, A. S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated inorganic nanoparticles. Angew. Chem., Int. Ed. 2011, 50, 1980–1994.
Stefanick, J. F.; Ashley, J. D.; Kiziltepe, T.; Bilgicer, B. A systematic analysis of peptide linker length and liposomal polyethylene glycol coating on cellular uptake of peptidetargeted liposomes. ACS Nano 2013, 7, 2935–2947.
Oishi, M.; Nakaogami, J.; Ishii, T.; Nagasaki, Y. Smart PEGylated gold nanoparticles for the cytoplasmic delivery of siRNA to induce enhanced gene silencing. Chem. Lett. 2001, 35, 1046–1047.
Lushchak, V. I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837.
Conde, J.; Tian, F. R.; Hernández, Y.; Bao, C. C.; Cui, D. X.; Janssen, K.-P.; Ibarra, M. R.; Baptista, P. V.; Stoeger, T.; de la Fuente, J. M. In vivo tumor targeting via nanoparticlemediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials 2013, 34, 7744–7753.
Cebrián, V.; Martín-Saavedra, F.; Yagüe, C.; Arruebo, M.; Santamaría, J.; Vilaboa, N. Size-dependent transfection efficiency of PEI-coated gold nanoparticles. Acta Biomater. 2011, 7, 3645–3655.
Dreaden, E. C.; Mackey, M. A.; Huang, X. H.; Kang, B.; El-Sayed, M. A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404.
Conde, J.; Ambrosone, A.; Sanz, V.; Hernandez, Y.; Marchesano, V.; Tian, F. R.; Child, H.; Berry, C. C.; Ibarra, M. R.; Baptista, P. V. et al. Design of multifunctional gold nanoparticles for in vitro and in vivo gene silencing. ACS Nano 2012, 6, 8316–8324.
Frens, G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22.
Turkevich, J.; Stevenson, P. C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 55–75.
Lee, P. C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395.
McBeath, R.; Pirone, D. M.; Nelson, C. M.; Bhadriraju, K.; Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495.
Conde, J.; Oliva, N.; Artzi, N. Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E1278–E1287.
Ackerson, C. J.; Sykes, M. T.; Kornberg, R. D. Defined DNA/nanoparticle conjugates. Proc. Natl. Acad. Sci. USA 2005, 102, 13383–13385.
Kumar, D.; Meenan, B. J.; Dixon, D. Glutathione-mediated release of Bodipy® from PEG cofunctionalized gold nanoparticles. Int. J. Nanomedicine 2012, 7, 4007–4022.
Chompoosor, A.; Han, G.; Rotello, V. M. Charge dependence of ligand release and monolayer stability of gold nanoparticles by biogenic thiols. Bioconjugate Chem. 2008, 19, 1342–1345.
Hong, R.; Han, G.; Fernández, J. M.; Kim, B.-J.; Forbes, N. S.; Rotello, V. M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079.
Han, G.; Chari, N. S.; Verma, A.; Hong, R.; Marin, C. T.; Rotello, V. M. Controlled recovery of the transcription of nanoparticle-bound DNA by intracellular concentrations of glutathione. Bioconjugate Chem. 2005, 16, 1356–1359.
Zhu, Z.-J.; Yeh, Y.-C.; Tang, R.; Yan, B.; Tamayo, J.; Vachet, R. W.; Rotello, V. M. Stability of quantum dots in live cells. Nat. Chem. 2011, 3, 963–968.
Li, D.; Li, G. P.; Guo, W. W.; Li, P. C.; Wang, E. K.; Wang, J. Glutathione-mediated release of functional plasmid DNA from positively charged quantum dots. Biomaterials 2008, 29, 2776–2782.
Meister, A.; Anderson, M. E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760.
Wu, G. Y.; Fan, Y.-Z.; Yang, S.; Lupton, J. R.; Turner, N. D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492.
Lei, X. G. In vivo antioxidant role of glutathione peroxidase: Evidence from knockout mice. Methods Enzymol. 2002, 347, 213–225.
Fang, Y.-Z.; Yang, S.; Wu, G. Y. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879.
Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208.
Kah, J. C. Y.; Wong, K. Y.; Neoh, K. G.; Song, J. H.; Fu, J. W. P.; Mhaisalkar, S.; Olivo, M.; Richard, C. J. Critical parameters in the pegylation of gold nanoshells for biomedical applications: An in vitro macrophage study. J. Drug Target. 2009, 17, 181–193.
Rana, T. M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 23–36.
Li, Z. H.; Rana, T. M. Molecular mechanisms of RNAtriggered gene silencing machineries. Acc. Chem. Res. 2012, 45, 1122–1131.
Unsworth, L. D.; Sheardown, H.; Brash, J. L. Protein resistance of surfaces prepared by sorption of end-thiolated poly(ethylene glycol) to gold: Effect of surface chain density. Langmuir 2005, 21, 1036–1041.
Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U. S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem., Int. Ed. 2010, 49, 6288–6308.
Hinterwirth, H.; Kappel, S.; Waitz, T.; Prohaska, T.; Lindner, W.; Lämmerhofer, M. Quantifying thiol ligand density of self-assembled monolayers on gold nanoparticles by inductively coupled plasma-mass spectrometry. ACS Nano 2013, 7, 1129–1136.
Häkkinen, H. The gold-sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
McCully, M., Hernandez, Y., Conde, J. et al. Significance of the balance between intracellular glutathione and polyethylene glycol for successful release of small interfering RNA from gold nanoparticles. Nano Res. 8, 3281–3292 (2015). https://doi.org/10.1007/s12274-015-0828-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-015-0828-5