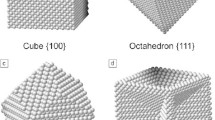
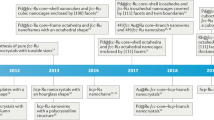
Abstract
Rhodium (Rh) is a critical component of many catalysts for a variety of chemical transformation processes. Controlling the shape of Rh nanocrystals offers an effective route to the optimization of their catalytic performance owing to a close correlation between the catalytic activity/selectivity and the surface atomic structure. It also helps to substantially reduce the loading amount and thus achieve a sustainable use of this scarce and precious metal. In this review article, we focus on recent progress in the shape-controlled synthesis of Rh nanocrystals with the goal of enhancing their catalytic properties. Both traditional and newly-developed synthetic strategies and growth mechanisms will be discussed, including those based on the use of surface capping agents, manipulation of reduction kinetics, control of surface diffusion rate, management of oxidation etching, and electrochemical alteration. We also use two examples to highlight the unique opportunities offered by shape-controlled synthesis for enhancing the use of this metal in catalytic applications. The strategies can also be extended to other precious metals in an effort to advance the production of cost-effective catalysts.

Similar content being viewed by others
References
Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282.
Dreaden, E. C.; Alkilany, A. M.; Huang, X. H.; Murphy, C. J.; El-Sayed, M. A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779.
Christopher, P.; Linic, S. Engineering selectivity in heterogeneous catalysis: Ag nanowires as selective ethylene epoxidation catalysts. J. Am. Chem. Soc. 2008, 130, 11264–11265.
Rycenga, M.; Cobley, C. M.; Zeng, J.; Li, W. Y.; Moran, C. H.; Zhang, Q.; Qin, D.; Xia, Y. N. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712.
Zhang, H.; Jin, M. S.; Xiong, Y. J.; Lim, B.; Xia, Y. N. Shapecontrolled synthesis of Pd nanocrystals and their catalytic applications. Acc. Chem. Res. 2013, 46, 1783–1794.
Chen, M.; Wu, B. H.; Yang, J.; Zheng, N. F. Small adsorbate-assisted shape control of Pd and Pt nanocrystals. Adv. Mater. 2012, 24, 862–879.
Peng, Z. M.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164.
Joo, S. H.; Park, J. Y.; Tsung, C.-K.; Yamada, Y.; Yang, P. D.; Somorjai, G. A. Thermally stable Pt/mesoporous silica core-shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131.
Quek, X.-Y.; Guan, Y. J.; Hensen, E. J. M. Structure sensitivity in the hydrogenation of unsaturated hydrocarbons over Rh nanoparticles. Catal. Today 2012, 183, 72–78.
Yuan, Y; Yan, N.; Dyson, P. J. Advances in the rational design of rhodium nanoparticle catalysts: control via manipulation of the nanoparticle core and stabilizer. ACS Catal. 2012, 2, 1057–1069.
Liguras, D. K.; Kondarides, D. I.; Verykios, X. E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal., B: Environ. 2003, 43, 345–354.
Campos-Skrobot, F. C.; Rizzo-Domingues, R. C. P.; Fernandes-Machado, N. R. C.; Cantão, M. P. Novel zeolite-supported rhodium catalysts for ethanol steam reforming. J. Power Sources 2008, 183, 713–716.
Zhang, Y. W.; Grass, M. E.; Huang, W. Y.; Somorjai, G. A. Seedless polyol synthesis and CO oxidation activity of monodisperse (111)- and (100)-oriented rhodium nanocrystals in sub-10 nm sizes. Langmuir 2010, 26, 16463–16468.
Wang, R.; He, H.; Wang, J. N.; Liu, L. C.; Dai, H. X. Shaperegulation: An effective way to control CO oxidation activity over noble metal catalysts. Catal. Today 2013, 201, 68–78.
Renzas, J. R.; Zhang, Y. W.; Huang, W. Y.; Somorjai, G. A. Rhodium nanoparticle shape dependence in the reduction of NO by CO. Catal. Lett. 2009, 132, 317–322.
Holles, J. H.; Switzer, M. A.; Davis, R. J. Influence of ceria and lanthana promoters on the kinetics of NO and N2O reduction by CO over alumina-supported palladium and rhodium. J. Catal. 2000, 190, 247–260.
Zhao, Z.-J.; Moskaleva, L. V.; Rösch, N. Ring-opening reactions of methylcyclopentane over metal catalysts, M = Pt, Rh, Ir, and Pd: A mechanistic study from first-principles calculations. ACS Catal. 2013, 3, 196–205.
Halasi, G.; Bánsági, T.; Solymosi, F. Production of hydrogen from dimethyl ether oversupported rhodium catalysts. ChemCatChem 2009, 1, 311–317.
Gandhi, H. S.; Graham, G. W.; McCabe, R. W. Automotive exhaust catalysis. J. Catal. 2003, 216, 433–442.
Loferski, P. J. Commodity Report: Platinum-Group Metal. United States Geological Survey, 2012-07-16.
Xie, S. F.; Choi, S.-I.; Xia, X.; Xia, Y. Catalysis on faceted noble-metal nanocrystals: both shape and size matter. Curr. Opin. Chem. Eng. 2013, 2, 142–150.
Gniewek, A.; Trzeciak, A. M. Rh(0) Nanoparticles: Synthesis, structure and catalytic application in Suzuki-Miyaura reaction and hydrogenation of benzene. Top Catal. 2013, 56, 1239–1245.
Xia, Y. N.; Xiong, Y. J.; Lim, B.; Skrabalak, S. E. Shapecontrolled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103.
Zou, Z. Y.; Tian, N.; Li, J. T.; Broadwell, I.; Sun, S. G. Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage. Chem. Soc. Rev. 2011, 40, 4167–4185.
Zhou, K. B.; Li, Y. D. Catalysis based on nanocrystals with well-defined facets. Angew. Chem. Int. Ed. 2012, 51, 602–613.
Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G. F.; Ross, P. N.; Lucas, C. A.; Markovic, N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497.
Zaera, F. Shape-controlled nanostructures in heterogeneous catalysis. ChemSusChem 2013, 6, 1797–1820.
Goodman, D. W. Model catalytic studies over metal single crystals. Acc. Chem. Res. 1984, 17, 194–200.
Li, Y. X.; Bowker, M. The adsorption and decomposition of nitrous oxide on Rh(110) and Rh(111). Surf. Sci. 1996, 348, 67–76.
Comelli, G.; Dhanak, V. R.; Kiskinova, M.; Prince, K. C.; Rosei, R. Oxygen and nitrogen interaction with rhodium single crystal surfaces. Surf. Sci. Rep. 1998, 32, 165–231.
Hopstaken, M. J. P.; Niemantsverdriet, J. W. Structure sensitivity in the CO oxidation on rhodium: Effect of adsorbate coverages on oxidation kinetics on Rh(100) and Rh(111). J. Chem. Phys. 2000, 113, 5457–5465.
Mavrikakis, M.; Bäumer, M.; Freund, H.-J.; Nørskov, J. K. Structure sensitivity of CO dissociation on Rh surfaces. Catal. Lett. 2002, 81, 153–156.
Hoefelmeyer, J. D.; Niesz, K.; Somorjai, G. A.; Tilley, T. D. Radial anisotropic growth of rhodium nanoparticles. Nano Lett. 2005, 5, 435–438.
Zettsu, N.; McLellan, J. M.; Wiley, B.; Yin, Y. D.; Li, Z.-Y.; Xia, Y. N. Synthesis, stability, and surface plasmonic properties of rhodium multipods, and their use as substrates for surface-enhanced Raman scattering. Angew. Chem. Int. Ed. 2006, 45, 1288–1292.
Park, K. H.; Jang, K.; Kim, H. J.; Son, S. U. Nearmonodisperse tetrahedral rhodium nanoparticles on charcoal: The shape-dependent catalytic hydrogenation of arenes. Angew. Chem. Int. Ed. 2007, 46, 1152–1155.
Zhang, Y. W.; Grass, M. E.; Habas, S. E.; Tao, F.; Zhang, T. F.; Yang, P. D.; Somorjai, G. A. One-step polyol synthesis and Langmuir-Blodgett monolayer formation of size-tunable monodisperse rhodium nanocrystals with catalytically active (111) surface structures. J. Phys. Chem. C 2007, 111, 12243–12253.
Humphrey, S. M.; Grass, M. E.; Habas, S. E.; Niesz, K.; Somorjai, G. A.; Tilley, T. D. Rhodium nanoparticles from cluster seeds: Control of size and shape by precursor addition rate. Nano Lett. 2007, 7, 785–790.
Biacchi, A. J.; Schaak, R. E. The solvent matters: kinetic versus thermodynamic shape control in the polyol synthesis of rhodium nanoparticles. ACS Nano 2011, 5, 8089–8099.
Xiong, Y. J.; Xia, Y. N. Shape-controlled synthesis of metal nanostructures: The case of palladium. Adv. Mater. 2007, 19, 3385–3391.
Kwon, S. G.; Hyeon, T. Colloidal chemical synthesis and formation kinetics of uniformly sized nanocrystals of metals, oxides, and chalcogenides. Acc. Chem. Res. 2008, 41, 1696–1709.
Xiong, Y. J.; McLellan, J. M.; Chen, J. Y.; Yin, Y. D.; Li, Z. Y.; Xia, Y. N. Kinetically controlled synthesis of triangular and hexagonal nanoplates of palladium and their SPR/SERS properties. J. Am. Chem. Soc. 2005, 127, 17118–17127.
Zeng, J.; Zhu, C.; Tao, J.; Jin, M. S.; Zhang, H.; Li, Z.-Y.; Zhu, Y. M.; Xia, Y. N. Controlling the nucleation and growth of silver on palladium nanocubes by manipulating the reaction kinetics. Angew. Chem. Int. Ed. 2012, 51, 2354–2358.
Wen, Y.-N; Zhang, H.-M. Surface energy calculation of the fcc metals by using the MAEAM. Solid State Commun. 2007, 144, 163–167.
Yu, N.-F.; Tian, N.; Zhou, Z.-Y.; Huang, L.; Xiao, J.; Wen, Y.-H.; Sun, S.-G. Electrochemical synthesis of tetrahexahedral rhodium nanocrystals with extraordinarily high surface energy and high electrocatalytic activity. Angew. Chem. Int. Ed. 2014, 53, 5097–5101.
Frenken, J. W. M.; Stoltze, P. Are vicinal metal surfaces stable? Phys. Rev. Lett. 1999, 82, 3500–3503.
Li, Y.; Huang, Y. Morphology-controlled synthesis of platinum nanocrystals with specific petides. Adv. Mater. 2010, 22, 1921–1925.
Zeng, J.; Zheng, Y. Q.; Rycenga, M.; Tao, J.; Li, Z.-Y.; Zhang, Q.; Zhu, Y. M.; Xia, Y. N. Controlling the shapes of silver nanocrystals with different capping agents. J. Am. Chem. Soc. 2010, 132, 8552–8553.
Gu, J.; Zhang, Y. W.; Tao, F. Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 2012, 41, 8050–8065.
Chiu, C.-Y.; Wu, H.; Yao, Z. Y.; Zhou, F; Zhang, H.; Ozolins, V.; Huang, Y. Facet-selective adsorption on noble metal crystals guided by electrostatic potential surfaces of aromatic molecules. J. Am. Chem. Soc. 2013, 135, 15489–15500.
Zhang, Y. W.; Grass, M. E.; Kuhn, J. N.; Tao, F.; Habas, S. E.; Huang, W. Y.; Yang, P.; Somorjai, G. A. Highly selective synthesis of catalytically active monodisperse rhodium nanocubes. J. Am. Chem. Soc. 2008, 130, 5868–5869.
Zhang, H.; Jin, M. S.; Liu, H. Y.; Wang, J. G.; Kim, M. J.; Yang, D. R.; Xie, Z. X.; Liu, J. Y.; Xia, Y. N. Facile synthesis of Pd-Pt alloy nanocages and their enhanced performance for preferential oxidation of CO in excess hydrogen. ACS Nano 2011, 5, 8212–8222.
Yao, S. Y.; Yuan, Y.; Xiao, C. X.; Li, W. Z.; Kou, Y.; Dyson, P. J.; Yan, N.; Asakura, H.; Teramura, K.; Tanaka, T. Insights into the formation mechanism of rhodium nanocubes. J. Phys. Chem. C 2012, 116, 15076–15086.
Jang, K.; Kim, H. J.; Son, S. U. Low-temperature synthesis of ultrathin rhodium nanoplates via molecular orbital symmetry interaction between rhodium precursors. Chem. Mater. 2010, 22, 1273–1275.
Duan, H. H.; Yan, N.; Yu, R.; Chang, C. R.; Zhou, G.; Hu, H. S.; Rong, H. P.; Niu, Z. Q; Mao, J. J.; Asakura, H.; et al. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, doi: 10.1038/ncomms4093.
Langille, M. R.; Personick, M. L.; Zhang, J.; Mirkin, C. A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554.
Zhang, H.; Jin, M. S.; Xia, Y. N. Nobel-metal nanocrystals with concave surfaces: Synthesis and applications. Angew. Chem. Int. Ed. 2012, 51, 7656–7673.
Zhang, H.; Li, W. Y.; Jin, M. S.; Zeng, J.; Yu, T. K.; Yang, D. R.; Xia, Y. N. Controlling the morphology of rhodium nanocrystals by manipulating the growth kinetics with a syringe pump. Nano Lett. 2011, 11, 898–903.
Xie, S. F.; Lu, N.; Xie, Z. X.; Wang, J. G.; Kim, M. J.; Xia, Y. N. Synthesis of Pd-Rh core-frame concave nanocubes and their conversion to Rh cubic nanoframes by selective etching of the Pd cores. Angew. Chem. Int. Ed. 2012, 51, 10266–10270.
Xia, X. H.; Xie, S. F.; Liu, M. C.; Peng, H. C.; Lu, N.; Wang, J. G.; Kim, M. J.; Xia, Y. N. On the role of surface diffusion in determining the shape or morphology of noblemetal nanocrystals. Proc. Natl. Acad. Sci. USA 2013, 110, 6669–6673.
Oura, K.; Lifshits, V. G.; Saranin, A. A.; Zotov, A. V.; Katayama, M. Surface Science: An Introduction. 1st Ed, Springer: Heidelberg, 2008; p 324.
Kolasinski, K. W. Surface Science: Foundations of Catalysis and Nanoscience; 2nd Ed, Wiley: New York, 2008.
Xie, S. F.; Zhang, H.; Lu, N.; Jin, M. S.; Wang, J. G.; Kim, M. J.; Xie, Z. X.; Xia, Y. N. Synthesis of rhodium concave tetrahedrons by collectively manipulating the reduction kinetics, facet-selective capping, and surface diffusion. Nano Lett. 2013, 13, 6262–6268.
Zheng, Y. Q.; Zeng, J.; Ruditskiy, A.; Liu, M. C.; Xia, Y. N. Oxidative etching and its role in manipulating the nucleation and growth of noble-metal nanocrystals. Chem. Mater. 2014, 26, 22–33.
Zhang, H.; Xia, X. H.; Li, W. Y.; Zeng, J.; Dai, Y. Q.; Yang, D. R.; Xia, Y. N. Facile synthesis of five-fold twinned, starfish-like rhodium nanocrystals by eliminating oxidative etching with a chloride-free precursor. Angew. Chem. Int. Ed. 2010, 49, 5296–5300.
Mulvihill, M. J.; Ling, X. Y.; Henzie, J.; Yang, P. D. Anisotropic etching of silver nanoparticles for plasmonic structures capable of single-particle SERS. J. Am. Chem. Soc. 2010, 132, 268–274.
Chen, Y. M.; Chen, Q. S.; Peng, S. Y.; Wang, Z. Q.; Lu, G.; Guo, G. C. Manipulating the concavity of rhodium nanocubes enclosed by high-index facets via site-selective etching. Chem. Commun. 2014, 50, 1662–1664.
Xie, S. F.; Peng, H. C.; Lu, N.; Wang, J. G.; Kim, M. J.; Xie, Z. X.; Xia, Y. N. Confining the nucleation and overgrowth of Rh to the {111} facets of Pd nanocrystal seeds: The roles of capping agent and surface diffusion. J. Am. Chem. Soc. 2013, 135, 16658–16667.
Chen. C.; Kang, Y. J.; Huo, Z.; Zhu, Z. W.; Huang, W. Y.; Xin, H. L.; Snyder, J. D.; Li, D. G.; Herron, J. A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Wang, Z. L. Transmission electron microscopy of shapecontrolled nanocrystals and their assemblies. J. Phys. Chem. B 2000, 104, 1153–1175.
Tian, N.; Zhou, Z. Y.; Sun, S. G.; Ding, Y.; Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735.
Xiao, J.; Liu, S.; Tian, N.; Zhou, Z.-Y.; Liu, H. X.; Xu, B. B.; Sun, S. G. Synthesis of convex hexoctahedral Pt micro/nanocrystals with high-index facets and electrochemistry-mediated shape evolution. J. Am. Chem. Soc. 2013, 135, 18754–18757.
Tian, N.; Zhou, Z. Y.; Yu, N. F.; Wang, L. Y.; Sun, S. G. Direct Electrodeposition of tetrahexahedral Pd nanocrystals with high-index facets and high catalytic activity for ethanol electrooxidation. J. Am. Chem. Soc. 2010, 132, 7580–7581.
Yoon, T. J.; Kim, J. I.; Lee, J. K. Rh-based olefin hydroformylation catalysts and the change of their catalytic activity depending on the size of immobilizing supporters. Inorg. Chim. Acta 2003, 345, 228–234.
Gustafson, J.; Westerström, R.; Mikkelsen, A.; Torrelles, X.; Balmes, O.; Bovet, N.; Andersen, J. N.; Baddeley, C. J.; Lundgren, E. Sensitivity of catalysis to surface structure: The example of CO oxidation on Rh under realistic conditions. Phys. Rev. 2008, 78, 045423.
Quer, X. Y.; Guan, Y. J.; Hensen, E. J. M. Structure sensitivity in the hydrogenation of unsaturated hydrocarbons over Rh nanoparticles. Catal. Today 2012, 183, 72–78.
Singh, U. K.; Vannice, M. A. Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts—a review. Appl. Catal. A: General 2001, 213, 1–24.
Housmans, T. H. M.; Feliu, J. M.; Koper, M. T. M. CO oxidation on stepped Rh[n (111) × (111)] single crystal electrodes: A voltammetric study. J. Electroanal. Chem. 2004, 572, 79–91.
Liu, H. Z.; Jiang, T.; Han, B. X.; Liang, S. G.; Zhou, Y. X. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst. Science 2009, 326, 1250–1252.
Franke, R.; Selent, D.; Börner, A. Applied hydroformylation. Chem. Rev. 2012, 112, 5675–5732.
Hou, C.; Zhao, G. F.; Ji, Y. J.; Niu, Z. Q.; Wang, D. S.; Li, Y. D. Hydroformylation of alkenes over rhodium supported on the metal-organic framework ZIF-8. Nano Res. 2014, 7, 1364–1369.
Huang, X. Q.; Zhao, Z. P.; Chen, Y.; Chiu, C. Y.; Ruan, L. Y.; Liu, Y.; Li, M. F.; Duan, X. F.; Huang, Y. High density catalytic hot spots in ultrafine wavy nanowires. Nano Lett. 2014, 14, 3887–3894.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, S., Liu, X.Y. & Xia, Y. Shape-controlled syntheses of rhodium nanocrystals for the enhancement of their catalytic properties. Nano Res. 8, 82–96 (2015). https://doi.org/10.1007/s12274-014-0674-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0674-x