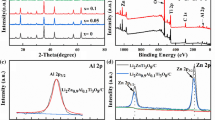
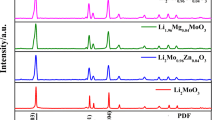
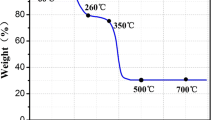
Abstract
Currently, many organic materials are being considered as electrode materials and display good electrochemical behavior. However, the most critical issues related to the wide use of organic electrodes are their low thermal stability and poor cycling performance due to their high solubility in electrolytes. Focusing on one of the most conventional carboxylate organic materials, namely lithium terephthalate Li2C8H4O4, we tackle these typical disadvantages via modifying its molecular structure by cation substitution. CaC8H4O4 and Al2(C8H4O4)3 are prepared via a facile cation exchange reaction. Of these, CaC8H4O4 presents the best cycling performance with thermal stability up to 570 °C and capacity of 399 mA·h·g−1, without any capacity decay in the voltage window of 0.005–3.0 V. The molecular, crystal structure, and morphology of CaC8H4O4 are retained during cycling. This cation-substitution strategy brings new perspectives in the synthesis of new materials as well as broadening the applications of organic materials in Li/Na-ion batteries.

Similar content being viewed by others
References
Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184.
Goodenough, J. B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603.
Armand, M.; Tarascon, J. M. Building better batteries. Nature 2008, 451, 652–657.
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
Li, C. S.; Zhang, S. Y.; Cheng, F. Y.; Ji, W. Q.; Chen, J. Porous LiFePO4/NiP composite nanospheres as the cathode materials in rechargeable lithium ion batteries. Nano Res. 2008, 1, 242–248.
Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J. M. From biomass to a renewable LixC6O6 organic electrode for sustainable Li-ion batteries. ChemSuschem 2008, 1, 348–355.
Luo, C.; Huang, R. M.; Kevorkyants, R.; Pavanello, M.; He, H. X.; Wang, C. S. Self-assembled organic nanowires for high power density lithium ion batteries. Nano Lett. 2014, 14, 1596–1602.
Walker, W.; Grugeon, S.; Mentre, O.; Laruelle, S.; Tarascon, J. M.; Wudl, F. Ethoxycarbonyl-based organic electrode for Li-batteries. J. Am. Chem. Soc. 2010, 132, 6517–6523.
Abouimrane, A.; Weng, W.; Eltayeb, H.; Cui, Y. J.; Niklas, J.; Poluektov, O.; Amine, K. Sodium insertion in carboxylate based materials and their application in 3.6 V full sodium cells. Energy Environ. Sci. 2012, 5, 9632–9638.
Liang, Y. L.; Tao, Z. L.; Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2012, 2, 742–769.
Zhang, H. Q.; Deng, Q. J.; Mou, C. X.; Huang, Z. L.; Wang, Y.; Zhou, A. J.; Li, J. Z. Surface structure and high-rate performance of spinel Li4Ti5O12 coated with N-doped carbon as anode material for lithium-ion batteries. J. Power Sources 2013, 239, 538–545.
Wang, S. W.; Wang, L. J.; Zhu, Z. Q.; Hu, Z.; Zhao, Q.; Chen, J. All organic sodium-ion batteries with Na4C8H2O6. Angew. Chem. Int. Ed. 2014, 53, 5892–5896.
Wang, S. W.; Wang, L. J.; Zhang, K.; Zhu, Z. Q.; Tao, Z. L.; Chen, J. Organic Li4C8H2O6 nanosheets for lithium-ion batteries. Nano Lett. 2013, 13, 4404–4409.
Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribière, P.; Poizot, P.; Tarascon, J. M. Conjugated dicarboxylate anodes for Li-ion batteries. Nat. Mater. 2009, 8, 120–125.
Nuzzo, R. G.; Zegarski, B. R.; Dubois, L. H. Fundamental studies of the chemisorption of organosulfur compounds on gold (111). Implications for molecular self-assembly on gold surfaces. J. Am. Chem. Soc. 1987, 109, 733–740.
Strong, L.; Whitesides, G. M. Structures of self-assembled monolayer films of organosulfur compounds adsorbed on gold single crystals: Electron diffraction studies. Langmuir 1988, 4, 546–558.
MacDiarmid, A. G. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590.
Nalwa, H. S. Handbook of Organic Conductive Molecules and Polymers, Conductive Polymers: Transport, Photophysics and Applications, Vol. 4. Wiley: New York, 1997.
Shirakawa, H.; Louis, E. J.; MacDiarmid, A. G.; Chiang, C. K.; Heeger, A. J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene (CH)x. J. Chem. Chem. Commun. 1977, 578–580.
Guo, W.; Yin, Y. X.; Xin, S.; Guo, Y. G.; Wan, L. J. Superior radical polymer cathode material with a two-electron process redox reaction promoted by graphene. Energy Environ. Sci. 2012, 5, 5221–5225.
Chiefari, J.; Chong, Y. K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T. P.; Mayadunne, R. T.; Meijs, G. F.; Moad, C. L.; Moad, G. et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562.
Wang, J. S.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom ttransfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615.
Zhang, Y. Y.; Sun, Y. Y.; Du, S. X.; Gao, H. J.; Zhang, S. B. Organic salts as super-high rate capability materials for lithium-ion batteries. Appl. Phys. Lett. 2012, 100, 091905.
Kaduk, J. A. Terephthalate salts: Salts of monopositive cations. Acta Cryst. 2000, B56, 474–485.
Zhao, L.; Zhao, J. M.; Hu, Y. S.; Li, H.; Zhou, Z. B.; Armand, M.; Chen, L. Q. Disodium terephthalate (Na2C8H4O4) as high performance anode material for low cost room temperature sodium ion battery. Adv. Energy Mater. 2012, 2, 962–965.
Park, Y. W.; Shin, D. S.; Woo, S. H.; Choi, N. S.; Shin, K. H.; Oh, S. M.; Lee, K. T.; Hong, S. Y. Sodium terephthalate as an organic anode material for sodium ion batteries. Adv. Mater. 2012, 24, 3562–3567.
Zhang, B.; Wang, X. J.; Li, H.; Huang, X. J. Electrochemical performances of LiFe1−x MnxPO4 with high Mn content. J. Power Sources 2011, 196, 6992–6996.
Dahn, J. R.; Zheng, T.; Liu, Y. H. Xue, J. S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593.
Wang, L. P.; Schnepp, Z.; Titirici, M. M. Rice husk-derived carbon anodes for lithium ion batteries. J. Mater. Chem. A 2013, 1, 5269–5273.
Chen, P. C.; Xu, J.; Chen, H. T.; Zhou, C. W. Hybrid silicon-carbon nanostructured composites as superior anodes for lithium ion batteries. Nano Res. 2011, 4, 290–296.
Fei, H. L.; Peng, Z. W.; Li, L.; Yang, Y.; Lu, W.; Samuel, E. L. G.; Fan, X. J.; Tour, J. M. Preparation of carbon-coated iron oxide nanoparticles dispersed on graphene sheets and applications as advanced anode materials for lithium-ion batteries. Nano Res. 2014, 7, 502–510.
Armstrong, M. J.; O’Dwyer, C.; Macklin, W. J.; Holmes, J. D. Evaluating the performance of nanostructured materials as lithium-ion battery electrodes. Nano Res. 2014, 7, 1–62.
He, Y.; Yu, X. Q.; Wang, Y. H.; Li, H.; Huang, X. J. Alumina-coated patterned amorphous silicon as the anode for a lithium-ion battery with high coulombic efficiency. Adv. Mater. 2011, 23, 4938–4941.
Li, S. L.; Li, A. H.; Zhang, R. A.; He, Y. Y.; Zhai, Y. J.; Xu, L. Q. Hierarchical porous metal ferrite ball-in-ball hollow spheres: General synthesis, formation mechanism, and high performance as anode materials for Li-ion batteries. Nano Res. 2014, 7, 1116–1127.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, L., Zhang, H., Mou, C. et al. Dicarboxylate CaC8H4O4 as a high-performance anode for Li-ion batteries. Nano Res. 8, 523–532 (2015). https://doi.org/10.1007/s12274-014-0666-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0666-x