Abstract
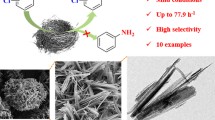
We report on the preparation of three kinds of Ni nanoparticles supported on carbon (Ni/C) and their application in the catalytic hydrolysis of ammonia borane (AB). Three Ni/C catalysts were prepared from a Ni metal-organic framework (Ni-MOF) precursor by reduction with KBH4, calcination at 700 °C under Ar, and a combination of calcination and reduction, the products being denoted as Ni/C-1, Ni/C-2, and Ni/C-3, respectively. The structure, morphology, specific surface area, and element valence were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption-desorption measurements, and X-ray photoelectron spectra (XPS). The results demonstrate that Ni/C-1 is composed of amorphous Ni particles agglomerated on carbon, Ni/C-2 is characteristic of crystalline Ni nanoparticles (about 10 nm in size) supported on carbon with Ni oxidized on the surface, while the surface of the Ni particles in Ni/C-3 is less oxidized. The specific surface areas of Ni-MOF, Ni/C-1, Ni/C-2, and Ni/C-3 are 1239, 33, 470, and 451 m2·g−1, respectively. The catalytic hydrolysis of AB with Ni/C-3 shows a hydrogen generation rate of 834 mL·min−1·g−1 at room temperature and an activation energy of 31.6 kJ/mol. Ni/C-3 shows higher catalytic activity than other materials, which can be attributed to its larger surface area of crystalline Ni. This study offers a promising way to replace noble metal by Ni nanoparticles for AB hydrolysis under ambient conditions.

Similar content being viewed by others
References
Grochala, W.; Edwards, P. P. Thermal decomposition of the non-intersticial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1316.
Peng, B.; Chen, J. Ammonia borane as an efficient and lightweight hydrogen storage medium. Energy Environ. Sci. 2008, 1, 479–483.
Graham, T. W.; Tsang, C.-W.; Chen, X. H.; Guo, R. W.; Jia, W. L.; Lu, S.-M.; Sui-Seng, C.; Ewart, C. B.; Lough, A.; Amoroso, D., et al. Catalytic solvolysis of ammonia borane. Angew Chem. Int. Ed. 2010, 49, 8708–8711.
Zhao, J. Z.; Shi, J. F.; Zhang, X. W.; Cheng, F. Y.; Liang, J.; Tao, Z. L.; Chen, J. A soft hydrogen storage material: Poly(methyl acrylate)-confined ammonia borane with controllable dehydrogenation. Adv. Mater. 2010, 22, 394–397.
Wang. P. Solid-state thermolysis of ammonia borane and related materials for high-capacity hydrogen storage. Dalton Trans. 2012, 41, 4296–4302.
Cheng. F. Y.; Ma, H.; Li, Y.; Chen, J. Ni1−x Ptx (x = 0–0.12) hollow spheres as catalysts for hydrogen generation from ammonia borane. Inorg. Chem. 2007, 46, 788–794.
Xu, Q.; Chandra, M. Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia-borane at room temperature. J. Power Sources 2006, 163, 364–370.
Akdim, O.; Demirci, U. B.; Miele, P. Deactivation and reactivation of cobalt in hydrolysis of sodium borohydrodide. Int. J. Hydrogen Energy 2011, 36, 13669–13675.
Liu, C.-H.; Wu, Y.-C.; Chou, C.-C.; Chen, B.-H.; Hsueh, C.-L.; Ku, J.-R.; Tsau, F. Hydrogen generated from hydrolysis of ammonia borane using cobalt and ruthenium based catalysts. Int. J. Hydrogen Energy 2012, 37, 2950–2959.
Song, P.; Li, Y. Q.; Li, W.; He, B.; Yang, J. Z.; Li, X. G. A highly efficient Co (0) catalyst derived from metal-organic framework for the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2011, 36, 10468–10473.
Yao, C. F.; Zhuang, L.; Cao, Y. L.; Ai, X. P.; Yang, H. X. Hydrogen release from hydrolysis of borazane on Pt- and Ni-based alloy catalysts. Int. J. Hydrogen Energy 2008, 33, 2462–2467.
Eom, K. S.; Kim, M. J.; Kim, R. H.; Nam, D. H.; Kwon, H. S. Characterization of hydrogen generation for fuel cells via borane hydrolysis using an electroless-deposited Co-P/Ni foam catalyst. J. Power Sources 2010, 195, 2830–2834.
Chen, D.; Li, J. J.; Shi, C. S.; Du, X. W.; Zhao, N. Q.; Sheng, J.; Liu, S. Properties of core-shell Ni-Au nanoparticles synthesized through a redox-transmetalation method in reverse microemulsion. Chem. Mater. 2007, 19, 3399–3405.
Metin, Ö.; Mazumder, V.; Özkar, S.; Sun, S. H. Monodisperse nickel nanoparticles and their catalysis in hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc. 2010, 132, 1468–1469.
Umegaki, T.; Yan, J.-M.; Zhang, X.-B.; Shioyama, H.; Kuriyama, N.; Xu, Q. Preparation and catalysis of poly(N-vinyl-2-pyrrolidone) (PVP) stabilized nickel catalyst for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2009, 34, 3816–3822.
Yan, J.-M.; Zhang, X.-B.; Han, S.; Shioyama, H.; Xu, Q. Synthesis of longtime water/air-stable Ni nanoparticles and their high catalytic activity for hydrolysis of ammonia-borane for hydrogen generation. Inorg. Chem. 2009, 48, 7389–7393.
Jiang, H. L.; Umegaki, T.; Akita, T.; Zhang, X. B.; Haruta, M.; Xu, Q. Bimetallic Au-Ni nanoparticles embedded in SiO2 nanospheres: Synergtic catalysis in hydrolytic dehydrogenation of ammonia borane. Chem. Eur. J. 2010, 16, 3132–3137.
Lai, S.-W.; Lin, H.-L.; Lin, Y.-P.; Yu, T. L. Hydrolysis of ammonia-borane catalyzed an iron-nickel alloy on an SBA-15 support. Int. J. Hydrogen Energy 2013, 38, 4636–4647.
Chandra, M.; Xu, Q. Dissociation and hydrolysis of ammonia-borane with solid acids and carbon dioxide: An efficient hydrogen generation system. J. Power Sources 2006, 159, 855–860.
Satyapal, S.; Read, C.; Ordaz, G.; Thomas, G. 2006 Annual DOE hydrogen program merit review: Hydrogen storage. Washington, D.C.: U.S. Department of Energy. http://www.hydrogenenergy.gov/pdfs/review06/2-storagesatyapal.pdf; 2006.
Rakap, M.; Kalu, E. E.; Özkar, S. Hydrogen generation from the hydrolysis of ammonia borane using cobalt-nickel-phosphorous (Co-Ni-P) catalyst supported on Pd-activated TiO2 by electroless deposition. Int. J. Hydrogen Energy 2011, 36, 254–261.
Li, Y. L.; Xie, L.; Liu, Y.; Yang, R.; Li, X. G. Favorable hydrogen storage properties of M(HBTC)(4,4′-bipy)·3DMF (M = Ni and Co). Inorg. Chem. 2008, 47, 10372–10377.
Zhao, J. Z.; Ma, H.; Cheng, J. Improved hydrogen generation from alkaline NaBH4 solution using carbon-supported Co-B as catalysts. Int. J. Hydrogen Energy 2007, 32, 4711–4716.
Aranishi, K.; Jiang, H.-L.; Akita, T.; Haruta, M.; Xu, Q. One-step synthesis of magnetically recyclable Au/Co/Fe triple-layered core-shell nanoparticles as highly efficient catalysts for the hydrolytic dehydrogenation of ammonia borane. Nano Res. 2011, 4, 1233–1241.
Dai, H.-B.; Liang, Y.; Wang, P. Effect of trapped hydrogen on the induction period of cobalt-tungsten-boron/nickel foam catalyst in catalytic hydrolysis reaction of sodium borohydride. Catal. Today 2011, 170, 27–32.
Yang, X. J.; Cheng, F. Y.; Liang, J.; Tao, Z. L.; Chen, J. Carbon-supported Ni1−x @Ptx (x = 0.32, 0.43, 0.60, 0.67, and 0.80) core-shell nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2011, 36, 1984–1990.
Metin, Ö.; Özkar, S.; Sun, S. H. Monodisperse nickel nanoparticles supported on SiO2 as an effective catalysts for the hydrolysis of ammonia-borane. Nano Res. 2010, 3, 676–684.
Yang, X. J.; Cheng, F. Y.; Liang, J.; Tao, Z. L.; Chen, J. PtxNi1−x nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2009, 34, 8785–8791.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, L., Zhang, T., Tao, Z. et al. Ni nanoparticles supported on carbon as efficient catalysts for the hydrolysis of ammonia borane. Nano Res. 7, 774–781 (2014). https://doi.org/10.1007/s12274-014-0438-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0438-7