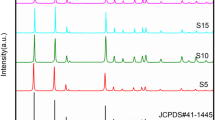
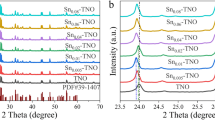
Abstract
[020]-oriented tin sulfide nanobelts with a length/thickness ratio of 100 have been synthesized by a facile hydrothermal method without any surfactants, and the nanobelts have shown good strain-accommodating properties as well as good electrochemical performance as the anode for Li-ion batteries. The formation of the nanobelts results from a precipitation-dissolution-transformation mechanism, and the [020] oriented growth can be ascribed to the {010} facet family having the lowest atomic density. In particular, SnS shows clear Li-Sn alloying/de-alloying reversible reactions in the potential range 0.1–1.0 V. Based on galvanostatic measurements and electrochemical impedance spectroscopy, SnS nanobelts have shown impressive rate performance. The post-cycled SnS nanobelts were completely transformed into metallic tin, and preserved the one-dimensional structure due to their flexibility which accommodates the large volumetric expansion.

Similar content being viewed by others
References
Sharon, M.; Basavaswaran, K. Photoelectrochemical behaviour of tin monosulphide. Solar Cells 1988, 25, 97–107.
Price, L. S.; Parkin, I. P.; Hardy, A. M. E.; Clark, R. J. H.; Hibbert, T. G.; Molloy, K. C. Atmospheric pressure chemical vapor deposition of tin sulfides (SnS, Sn2S3, and SnS2) on glass. Chem. Mater. 1999, 11, 1792–1799.
Rajeshwar, K.; de Tacconi, N. R.; Chenthamarakshan, C. R. Semiconductor-based composite materials: Preparation, properties, and performance. Chem. Mater. 2001, 13, 2765–2782.
Chen, D.; Shen, G. Z.; Tang, K. B.; Lei, S. J.; Zheng, H. G.; Qian, Y. T. Microwave-assisted polyol synthesis of nanoscale SnSx [x = 1, 2] flakes. J. Cryst. Growth 2004, 260, 469–474.
Nassary, M. M. Temperature dependence of the electrical conductivity, Hall effect and thermoelectric power of SnS single crystals. J. Alloy. Compd. 2005, 398, 21–25.
Boonsalee, S.; Gudavarthy, R. V.; Bohannan, E. W.; Switzer, J. A. Epitaxial electrodeposition of tin(II) sulfide nanodisks on single-crystal Au(100). Chem. Mater. 2008, 20, 5737–5742.
Hayashi, A.; Konishi, T.; Tadanaga, K.; Minami, T.; Tatsumisago, M. All-solid-state lithium secondary batteries with SnS-P2S5 negative electrodes and Li2S-P2S5 solid electrolytes. J. Power Sources 2005, 146, 496–500.
Li, Y.; Tu, J. P.; Huang, X. H.; Wu, H. M.; Yuan, Y. F. Nanoscale SnS with and without carbon-coatings as an anode material for lithium ion batteries. Electrochim. Acta 2006, 52, 1383–1389.
Li, Y.; Tu, J. P.; Huang, X. H.; Wu, H. M.; Yuan, Y. F. Net-like SnS/carbon nanocomposite film anode material for lithium ion batteries. Electrochem. Commun. 2007, 9, 49–53.
Aso, K.; Hayashi, A.; Tatsumisago, M. Synthesis of needlelike and platelike SnS active materials in high-boiling solvents and their application to all-solid-state lithium secondary batteries. Cryst. Growth Des. 2011, 11, 3900–3904.
Zhang, Y. J.; Lu, J.; Shen, S. L.; Xu, H. R.; Wang, Q. B. Ultralarge single crystal SnS rectangular nanosheets. Chem. Commun. 2011, 47, 5226–5228.
Hegde, S. S.; Kunjomana, A. G.; Chandrasekharan, K. A.; Ramesh, K.; Prashantha, M. Optical and electrical properties of SnS semiconductor crystals grown by physical vapor deposition technique. Physica B 2011, 406, 1143–1148.
Yang, J.; Winter, M.; Besenhard, J. O. Small particle size multiphase Li-alloy anodes for lithium-ion-batteries. Solid State Ionics 1996, 90, 281–287.
Gou, X. L.; Chen, J.; Shen, P. W. Synthesis, characterization and application of SnSx (x = 1, 2) nanoparticles. Mater. Chem. Phys. 2005, 93, 557–566.
Park, M. S.; Wang, G. X.; Kang, Y. M.; Wexler, D.; Dou, S. X.; Liu, H. K. Preparation and electrochemical properties of SnO2 nanowires for application in lithium-ion batteries. Angew. Chem. Int. Ed. 2007, 46, 750–753.
Chan, C. K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X. F.; Huggins, R. A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35.
Wang, Z.; Luan, D.; Madhavi, S.; Li, M. C.; Lou, W. X. α-Fe2O3 nanotubes with superior lithium storage capability. Chem. Commun. 2011, 47, 8061–8063.
Kim, D. K.; Muralidharan, P.; Lee, H. W.; Ruffo, R.; Yang, Y.; Chan, C. K.; Peng, H.; Huggins, R. A.; Cui, Y. Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett. 2008, 8, 3948–3952.
Kim, M. G.; Jo, M.; Hong, Y.-S.; Cho, J. Template-free synthesis of Li[Ni0.25Li0.15Mn0.6]O2 nanowires for high performance lithium battery cathode. Chem. Commun. 2009, 218–220.
Lim, S. Y.; Yoon, C. S.; Cho, J. P. Synthesis of nanowire and hollow LiFePO4 cathodes for high-performance lithium batteries. Chem. Mater. 2008, 20, 4560–4564.
Li, X. X.; Cheng, F. Y.; Guo, B.; Chen, J. Template- synthesized LiCoO2, LiMn2O4, and LiNi0.8Co0.2O2 nanotubes as the cathode materials of lithium ion batteries. J. Phys. Chem. B 2005, 109, 14017–14024.
Liu, J.; Xue, D. Sn-based nanomaterials converted from SnS nanobelts: Facile synthesis, characterizations, optical properties and energy storage performances. Electrochim. Acta 2010, 56, 243–250.
Panda, S. K.; Datta, A.; Dev, A.; Gorai, S.; Chaudhuri, S. Surfactant-assisted synthesis of SnS nanowires grown on tin foils. Cryst. Growth Des. 2006, 6, 2177–2181.
Biswas, S.; Kar, S.; Chaudhuri, S. Thioglycolic. acid (TGA) assisted hydrothermal synthesis of SnS nanorods and nanosheets. Appl. Surf. Sci. 2007, 253, 9259–9266.
Dhanaraj, G.; Byrappa, K.; Prasad, V.; Dudley, M. Springer Handbook of Crystal Growth; Springer-Verlag: Berlin Heidelberg, 2010.
Francis, R. J.; Price, S. J.; Evans, J. S. O.; O’Brien, S.; O’Hare, D.; Clark, S. M. Hydrothermal synthesis of microporous tin sulfides studied by real-time in situ energy-dispersive X-ray diffraction. Chem. Mater. 1996, 8, 2102–2108.
Mariano, A. N.; Chopra, K. L. Polymorphism in some IV–VI compounds induced by high pressure and thin-film epitaxial growth. Appl. Phys. Lett. 1967, 10, 282–284.
Kim, D.; Shimpi, P.; Gao, P. X. Zigzag zinc blende ZnS nanowires: Large scale synthesis and their structure evolution induced by electron irradiation. Nano Res. 2009, 2, 966–974.
Hickey, S. G.; Waurisch, C.; Rellinghaus, B.; Eychmuller, A. Size and shape control of colloidally synthesized IV–VI nanoparticulate tin(II) sulfide. J. Am. Chem. Soc. 2008, 130, 14978–14980.
Tanusevski, A. Optical and photoelectric properties of SnS thin films prepared by chemical bath deposition. Semicond. Sci. Technol. 2003, 18, 501–505.
Liu, H. T.; Liu, Y.; Wang, Z.; He, P. Facile synthesis of monodisperse, size-tunable SnS nanoparticles potentially for solar cell energy conversion. Nanotechnology 2010, 21, 105707.
Seo, J. W.; Jang, J. T.; Park, S. W.; Kim, C. J.; Park, B. W.; Cheon, J. W. Two-dimensional SnS2 nanoplates with extraordinary high discharge capacity for lithium ion batteries. Adv. Mater. 2008, 20, 4269–4273.
Zhang, W. -M.; Hu, J. -S.; Guo, Y. -G.; Zheng, S. -F.; Zhong, L. -S. Song, W. -G.; Wan, L. -J. Tin-nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in lithium-ion batteries. Adv. Mater. 2008, 20, 1160–1165.
Liu, S.; Yin, X. M.; Chen, L. B.; Li, Q. H.; Wang, T. H. Synthesis of self-assembled 3D flowerlike SnS2 nanostructures with enhanced lithium ion storage property. Solid State Sci. 2010, 12, 712–718.
Huggins, R. A. Lithium alloy negative electrodes. J. Power Sources 1999, 81–82, 13–19.
Idota, Y.; Kubota, T.; Matsufuji, A.; Maekawa, Y.; Miyasaka, T. Tin-based amorphous oxide: A high-capacity lithium- ion-storage material. Science 1997, 276, 1395–1397.
Besenhard, J. O. Handbook of Battery Materials; Wiley-VCH: Weinheim, 1999.
Courtney, I. A.; Dahn, J. R. Key factors controlling the reversibility of the reaction of lithium with SnO2 and Sn2BPO6 glass. J. Electrochem. Soc. 1997, 144, 2943–2948.
Lou, X. W.; Wang, Y.; Yuan, C.; Lee, J. Y.; Archer, L. A. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lu, J., Nan, C., Li, L. et al. Flexible SnS nanobelts: Facile synthesis, formation mechanism and application in Li-ion batteries. Nano Res. 6, 55–64 (2013). https://doi.org/10.1007/s12274-012-0281-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-012-0281-7