Abstract
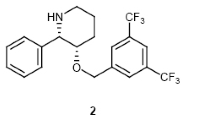
The first asymmetric synthesis of (R,R)-clemastine (1) has been accomplished by the coupling of (R)-tertiary alcohol 2 and (R)-chloroethylpyrrolidine 3 via O-alkylation. (R)-Tertiary alcohol 2 was synthesized by stereoselective alkylation of chiral α-benzyloxy ketone with Grignard reagent via chelation-controlled 1,4-asymmetric induction. In the reaction, chiral benzyl group acts as a chiral auxiliary as well as a protecting group. (R)-Chloroethylpyrrolidine 3 was prepared by asymmetric transformation starting with l-homoserine lactone, in which racemization-minimized N-allylation and ring-closing metathesis were involved as key steps.




Similar content being viewed by others
References
Chang, M., T.H. Kim, and H.D. Kim. 2008. Stereoselective synthesis of (+)-flutriafol. Tetrahedron Asymmetry 19: 1503–1507.
Ebnöther, A., and H.P. Weber. 1976. Synthesis and absolute configuration of clemastine and its isomers. Helvetica Chimica Acta 59: 2462–2468.
Fernández-Mateos, E., B. Marciá, and M. Yus. 2014. Catalytic enantioselective addition of aryl Grignard reagents to ketones. European Journal of Organic Chemistry 29: 6519–6526.
Forrat, V.J., O. Prieto, D.J. Ramón, and M. Yus. 2006. trans-1-Sufonylamino-2-isoborneolsulfonylaminocyclohexane derivatives: Excellent chiral ligands for catalytic enantioselective addition of organozinc reagents to ketones. Chemistry-A European Journal 12: 4431–4445.
Fournier, A.M., R.A. Brown, W. Farnaby, H. Miyatake-Ondozabal, and J. Clayden. 2010. Synthesis of (–)-(S,S)-clemastine by invertive N → C aryl migration in lithiated carbamate. Organic Letters 12: 2222–2225.
Han, P., R. Wang, and D.Z. Wang. 2011. Electronic polarizability-based stereochemical model for Sharpless AD reactions. Tetrahedron 67: 8873–8878.
Jung, J.E., H. Ho, and H.D. Kim. 2000. Bidentate chelation-controlled asymmetric synthesis of α-hydroxy esters based on the glycolate enolate alkylation. Tetrahedron Letters 41: 1793–1796.
Jung, J.W., and H.D. Kim. 2007. Stereoselective synthesis of (–)-hydroxyclemastine as a versatile intermediate for the H1 receptor antagonist clemastine. Archives of Pharmacal Research 30: 1521–1525.
Kim, S.J., M. Chang, and H.D. Kim. 2011. Asymmetric transformation of l-homoserine lactone to an optically active 2-substituted pyrrolidine for clemastine. Tetrahedron Asymmetry 22: 1901–1905.
Kim, T.H., Y.K. Kim, Z. Yang, J.W. Jung, L.S. Jeong, and H.D. Kim. 2014. A chiral benzyl group as a chiral auxiliary and protecting group for the synthesis of optically active 1,2-diols and (+)-frontalin. Synlett 25: 251–254.
Nelson, W.L. 2002. Antihistamines and related antiallergic and antiulcer agents. In Foye’s principles of medicinal chemistry, ed. D.A. Williams, and L.L. Thomas, 794–818. Philadelphia: Lippincott Williams & Wilkins.
Nikiforov, T., S. Stanchev, B. Milenkov, and V. Dimitrov. 1990. A route to optically active benzyl ester of (1-methyl-2-pyrrolidinyl)acetic acid, an intermediate in the synthesis of clemastine. Synthetic Communications 20: 1977–1981.
Sato, F., Y. Kobayashi, O. Takahashi, T. Chiba, Y. Takeda, and M. Kusakabe. 1985. Synthesis of the rarely obtained syn-adducts in the reaction of organocopper compounds with 2,3-O-isopropylideneglyceraldehyde. Preparation of optically active epoxy alcohols. Journal of the Chemical Society, Chemical Communications 22: 1636–1638.
Shimizu, K., H. Uetsu, G. Takahashi, and K. Ito. 2015. Zn(salen)-catalyzed enantioselective phenyl transfer to aldehydes and ketones with organozinc reagent. Synlett 26: 1238–1242.
Stymiest, J.L., V. Bagutski, R.M. French, and V.K. Aggarwal. 2008. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456: 778–783.
Takaoka, M. 1978. Optical resolution of clemastine. Japanese Patent JP53012857.
Vanhessche, K.P.M., and K.B. Sharpless. 1996. Ligand-dependent reversal of facial selectivity in asymmetric dihydroxylation. Journal of Organic Chemistry 61: 7978–7979.
Acknowledgments
This research was supported by the Sookmyung Women’s University Research Grants 2012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest with any financial, personal or other relationships with other people or organizations that could inappropriately influence or be perceived to influence this study.
Rights and permissions
About this article
Cite this article
Lee, S.Y., Jung, J.W., Kim, TH. et al. Asymmetric synthesis of H1 receptor antagonist (R,R)-clemastine. Arch. Pharm. Res. 38, 2131–2136 (2015). https://doi.org/10.1007/s12272-015-0641-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0641-4