Abstract
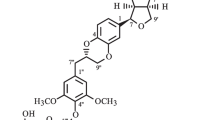
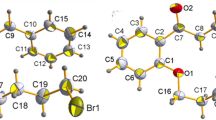
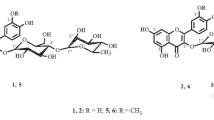
Two lignan glycosides, acanthoside D (1) (=liriodendrin, (+)-syringaresinol di-O-β-d-glucopyranoside) and eleutheroside E (2) have been confused each other for so long time, and hard to be distinguished each other. Now, this two compounds need to be defined properly so that all the commercial mistakes and confusions should not be made. They have identical planar structures except for the configurations at C-7 and C-8 in each structure according to the chemistry database, SciFinder®. The systematic name of acanthoside D is [(1S,3aR,4S,6aR)-tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1-phenylene) bis-β-d-glucopyranoside (1), and the name of eleutheroside E is [(1R,3aR,4S,6aS)-tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1-phenylene) bis-β-d-glucopyranoside (2). The differences at two chiral centers do not make any differences in the NMR spectra. Thus, the circular dichroism were utilized to dissolve this difficult problem. Acanthoside D (1) showed a positive Cotton effect at 200 nm, whereas eleutheroside E (2) exhibited a negative cotton effect at 200 nm. The absolute structure of acanthoside D was also confirmed by X-ray crystallography.





Similar content being viewed by others
References
Abe, F., and T. Yamauchi. 1988. Studies on Allamanda. Part 3. 9α-Hydroxypinoresinol, 9α-hydroxymedioresinol and related lignans from Allamanda neriifolia. Phytochemistry 27: 575–577.
Briggs, L.H., R.C. Cambie, and R.A.F. Couch. 1968. Lirioresinol-C dimethyl ether, a diaxially substituted 3,7-dioxabicyclo[3.3.0]octane lignan from Macropiper excelsum. Journal of the Chemical Society C 24: 3042–3045.
Cai, X.F., I.S. Lee, N.T. Dat, G. Shen, J.S. Kang, D.H. Kim, and Y.H. Kim. 2004. Inhibitory lignans against NFAT transcription factor from Acanthopanax koreanum. Archives of Pharmacal Research 27: 738–741.
Deyama, T. 1983. The constituents of Eucommia ulmoides Oliv. I. Isolation of (+)-medioresinol di-O-β-d-glucopyranoside. Chemical & Pharmaceutical Bulletin 31: 2993–2997.
Deyama, T., S. Nishibe, and Y. Nakazawa. 2001. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacologica Sinica 22: 1057–1070.
Elyakova, L.A., A.K. Dzizenko, and G.B. Elyakov. 1965. Structure of lignan glycosides from Acanthopanax sessiliflorus roots. Doklady Akademii Nauk SSSR 165: 562–565.
Elyakova, L.A., and G.B. Elyakov. 1965. Glycosides from Acanthopanax sessiliflorum roots, 555–556. Seriya Khimicheskaya: Izvestiya Akademii Nauk SSSR.
Feng, S., S. Ni, and W. Sun. 2007. Preparative isolation and purification of the lignan pinoresinol diglucoside and liriodendrin from the bark of Eucommia ulmoides Oliv. by high speed countercurrent chromatography. Journal of Liquid Chromatography & Related Technologies 30: 135–145.
Hofer, O., and R. Schoelm. 1981. Stereochemistry of tetrahydrofurofuran derivatives. Circular dichroism and absolute conformation. Tetrahedron 37: 1181–1186.
Jolad, S.D., J.J. Hoffmann, J.R. Cole, M.S. Tempesta, and R.B. Bates. 1980. Cytotoxic agent from Penstemon deustus (Scrophulariaceae): isolation and stereochemistry of liriodendrin, a symmetrically substituted furofuranoid lignan diglucoside. Journal of Organic Chemistry 45: 1327–1329.
Kiem, P.V., C.V. Minh, N.T. Dat, X.F. Cai, J.J. Lee, and Y.H. Kim. 2003. Two new phenylpropanoid glycosides from the stem bark of Acanthopanax trifoliatus. Archives of Pharmacal Research 26: 1014–1017.
Lami, N., S. Kadota, T. Kikuchi, and Y. Momose. 1991. Constituents of the roots of Boerhaavia diffusa L. III. Identification of calcium channel antagonistic compound from the methanol extract. Chemical & Pharmaceutical Bulletin 39: 1551–1555.
Li, T., B. Xu, J. Bai, K. Liu, and Y. Jiang. 2011. Chemical constituents from branches of Daphne genkwa. Zhongcaoyao 42: 1702–1705.
Nam, J.-W., S.-Y. Kim, T. Yoon, Y.J. Lee, Y.-S. Kil, Y.-S. Lee, and E.-K. Seo. 2013. Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents. Chemistry & Biodiversity 10: 1322–1327.
Ovodov, Y.S., G.M. Frolova, L.A. Elyakova, and G.B. Elyakov. 1965a. Identity of eleuteroside E with acanthoside D, 2065–2067. Seriya Khimicheskaya: Izvestiya Akademii Nauk SSSR.
Ovodov, Y.S., G.M. Frolova, M.Y. Nefedova, and G.B. Elyakov. 1967. Glycosides of Eleutherococcus senticosus. II. The structure of eleutherosides A, B1, C, and D. Khimiya Prirodnykh Soedinenii 3: 63–64.
Ovodov, Y.S., R.G. Ovodova, T.F. Solov’eva, G.B. Elyakov, and N.K. Kochetkov. 1965b. Glycosides from Eleutherococcus. I. Isolation and some properties of eleutherosides B and E. Khimiya Prirodnykh Soedinenii 1: 3–7.
Wang, Z., L. Zhang, and Y. Sun. 2005. Semipreparative separation and determination of eleutheroside E in Acanthopanax giraldii harms by high-performance liquid chromatography. Journal of Chromatographic Science 43: 249–252.
Yamazaki, T., S. Shimosaka, H. Sasaki, T. Matsumura, T. Tukiyama, and T. Tokiwa. 2007. (+)-Syringaresinol-di-O-β-D-glucoside, a phenolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SW982 human synovial sarcoma cells by inhibiting activating protein-1 and/or nuclear factor-κB activities. Toxicology in Vitro 21: 1530–1537.
Zhang, X.-Q., Y.-C. Lai, L. Wang, F.-F. Xu, M.-H. Gao, H. Li, Y.-L. Li, and W.-C. Ye. 2011. Phenylpropanoid constituents from Acanthopanax senticosus. Biochemical Systematics and Ecology 39: 861–863.
Zhang, X., H. Wu, C. Wu, P. Guo, X. Xu, and M. Yang. 2013. Pandanusphenol A and B: Two new phenolic compounds from the fruits of Pandanus tectorius Soland. Records of Natural Products 7: 359–362.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT and Future Planning (MSIF) (NRF-2013R1A2A2A01067336), by a Grant (13172MFDS417) from Ministry of Food and Drug Safety in 2013, and by a grant (12172MFDS989) from Ministry of Food and Drug Safety in 2014.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kil, YS., Park, JY., Kim, Y. et al. Utilization of circular dichroism experiment to distinguish acanthoside D and eleutheroside E. Arch. Pharm. Res. 38, 1921–1925 (2015). https://doi.org/10.1007/s12272-015-0586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0586-7