Abstract
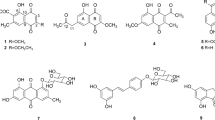
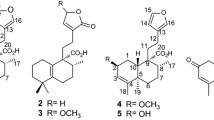
A new geranylated xanthone derivative, fuscaxanthone I (1), along with nine xanthones (2–9 and 11), a biphenyl (10) and three biflavonoids (12–14) were isolated from the roots of Garcinia fusca Pierre. Compounds 8, 10 and 11–14 were reported from this plant species for the first time. Their structures were elucidated by spectroscopic analyses, including 1D- and 2D-NMR and MS. The isolated compounds were evaluated for antibacterial activity against Helicobacter pylori. Cowaxanthone (5) and fukugiside (14) exhibited stronger inhibitory activity against H. pylori DMST reference strain at MICs 4.6 and 10.8 μM, respectively, than that of the control metronidazole. Isojacareubin (8) displayed the most potent activity against H. pylori HP40 clinical isolate with MIC 23.9 μM, which was approximately two times greater than that of the standard drug amoxicillin.


Similar content being viewed by others
References
Chen, F.-C., Y.-M. Lin, and J.-C. Hung. 1975. Phenolic compounds from the heartwood of Garcinia multiflora. Phytochemistry 14: 300–303.
Chey, W.D., and B.C.Y. Wong. 2007. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. American Journal of Gastroenterology 102: 1808–1825.
Chin, Y.-W., and A.D. Kinghorn. 2008. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini-Reviews in Organic Chemistry 5: 355–364.
Elfita, E., M. Muharni, M. Latief, D. Darwati, A. Widiyantoro, S. Supriyatna, H.H. Bahti, D. Dachriyanus, P. Cos, L. Maes, K. Foubert, S. Apers, and L. Pieters. 2009. Antiplasmodial and other constituents from four Indonesian Garcinia spp. Phytochemistry 70: 907–912.
Frahm, A.W., and R.K. Chaudhuri. 1979. 13C NMR spectroscopy of substituted xanthones II. 13C NMR spectral study of polyhydroxy xanthones. Tetrahedron 35: 2035–2038.
Hasegawa, H., S. Sakai, N. Aimi, H. Takayama, and T. Koyano. 1996 Helicabacter pylori inhibitors containing xanthones from Garcinia mangostana. Japan Kokai Tokkyo Koho, JP 08231396.
Ito, C., M. Itoigawa, T. Takakura, N. Ruangrungsi, F. Enjo, H. Tokuda, H. Nishino, and H. Furukawa. 2003. Chemical constituents of Garcinia fusca: Structure elucidation of eight new xanthones and their cancer chemopreventive activity. Journal of Natural Products 66: 200–205.
Konoshima, M., Y. Ikeshiro, A. Nishinaga, T. Matsuura, T. Kubota, and H. Sakamoto. 1969. The constitution of flavonoids from Garcinia spicata Hook. f. Tetrahedron Letters 10: 121–124.
Konoshima, M., and Y. Tkeshiro. 1970. Fukugiside, the first biflavonoid glycoside from Garcinia spicata Hook. f. Tetrahedron Letters 11: 1717–1720.
Li, X.-C., A.S. Joshi, B. Tan, H.N. ElSohly, L.A. Walker, J.K. Zjawiony, and D. Ferreira. 2002. Absolute configuration, conformation, and chiral properties of flavanone-(3->8′′)-flavone biflavonoids from Rheedia acuminata. Tetrahedron 58: 8709–8717.
Na Pattalung, P., W. Thongtheeraparp, P. Wiriyachitra, and W.C. Taylor. 1994. Xanthones of Garcinia cowa. Planta Medica 60: 365–366.
NCCLS (National Committee for Clinical Laboratory Standards). 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grows aerobically. Approved standards: M7-A4 and supplement tables M100-S7, 4th ed. Wayne: NCCLS.
Peres, V., T.J. Nagem, and F.F. Oliveira. 2000. Tetraoxygenated naturally occurring xanthones. Phytochemistry 55: 683–710.
Panthong, K., N. Hutadilok-Towatana, F. Cowaxanthone, and A. Panthong. 2009. A new tetraoxygenated xanthone, and other anti-inflammatory and anti-oxidant compounds from Garcinia cowa. Canadian Journal of Chemistry 87: 1636–1640.
Poomipamorn, S., and A. Kumkong. 1997. Edible multipurpose tree species. Bangkok: Faung Fa Printing.
Rath, G., O. Potterat, S. Mavi, and K. Hostettmann. 1996. Xanthones from Hypericum roeperanum. Phytochemistry 43: 513–520.
Rukachaisirikul, V., K. Tadpetch, A. Watthanaphanit, N. Saengsanae, and S. Phongpaichit. 2005. Benzopyran, biphenyl, and tetraoxygenated xanthone derivatives from the twigs of Garcinia nigrolineata. Journal of Natural Products 68: 1218–1221.
Suksamrarn, S., N. Suwannapoch, W. Phakhodee, J. Thanuhiranlert, P. Ratananukul, N. Chimnoi, and A. Suksamrarn. 2003. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chemical & Pharmaceutical Bulletin 51: 857–859.
Suksamrarn, S., O. Komutiban, P. Ratananukul, N. Chimnoi, N. Lartpornmatulee, and A. Suksamrarn. 2006. Cytotoxic prenylated xanthones from the young fruits of Garcinia mangostana. Chemical & Pharmaceutical Bulletin 54: 301–305.
Wang, Y.-C., and T.-L. Huang. 2005. Screening of anti-Helicobacter pylori herbs deriving from Taiwanese folk medicinal plants. FEMS Immunology & Medical Microbiology 43: 295–300.
Wangchuk, P., P.A. Keller, S.G. Pyne, M. Taweechotipatr, A. Tonsomboon, R. Rattanajak, and S. Kamchonwongpaisan. 2011. Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. Journal of Ethnopharmacology 137: 730–742.
Acknowledgments
This work was supported by the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission and Srinakharinwirot University (Grant number 231/2554). Authors thank Mr. Maurice Broughton, Chulabhorn Research Institute, Thailand, for proof reading the article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nontakham, J., Charoenram, N., Upamai, W. et al. Anti-Helicobacter pylori xanthones of Garcinia fusca . Arch. Pharm. Res. 37, 972–977 (2014). https://doi.org/10.1007/s12272-013-0266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0266-4