Abstract
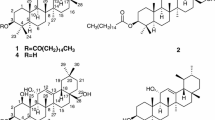
Three new nor-dammarane triterpenoids, 12β-O-acetyl-15α-hydoxy-3-oxo-17-en-20,21,22-23,24,25,26,27-octanordammanran (1), 12β,28-O-diacetyl-15α-hydoxy-3-oxo-17-en-20,21,22-23,24,25,26,27-octanordammanran (2), 12β-hydoxy-3,15-dioxo-20,21,22-23,24,25,26,27-octanordammanran (3), together with one known compound, 12β-O-acetyl-15α,28-dihydoxy-3-oxo-17-en-20,21,22-23,24,25,26,27-octanordammanran (4), were isolated from the 95 % EtOH extract of Dysoxylum hainanense. The structures of the new compounds were elucidated by spectral methods. All the triterpenoids were in vitro evaluated for their cytotoxic activities against four tumor cell lines (BGC-823, U251, HepG2 and SGC-7901). All the three nor-dammarane triterpenoids exhibited particular significant cytotoxic activities against glioma cell line.


Similar content being viewed by others
References
Albersberg, W., and Y. Singh. 1991. Dammarane triterpenoids from the fruits of Dysoxylum richii. Phytochemistry 30: 921–926.
Fujioka, T., A. Sakurai, K. Mihashi, Y. Kashiwada, I.S. Chen, and K.H. Lee. 1997. Antitumor agents. 168. Dysoxylum cumingianum. IV. The structures of cumingianosides G-O, new triterpene glucosides with a 14,18-cycloapotirucallane-type skeleton from Dysoxylum cumingianum, and their cytotoxicity against human cancer cell lines. Chemical and Pharmaceutical Bulletin 45: 68–74.
Govindachari, T.R., K.G.N. Kumari, and G. Suresh. 1997. Ergosta-5,24(24′)-diene-3β,4β,20S-triol, an ergostane steroid from Dysoxylum malabaricum. Phytochemistry 44: 153–155.
Govindachari, T.R., G. Suresh, and K.G.N. Kumari. 1994. Triterpenoids from Dysoxylum malabaricum. Phytochemistry 37: 1127–1129.
He, X.F., X.N. Wang, S. Yin, L. Dong, and J.M. Yue. 2011. Ring A-seco triterpenoids with antibacterial activity from Dysoxylum hainanense. Bioorganic and Medicinal Chemistry Letters 21: 125–129.
Jogia, M.K., and R.J. Andersen. 1987. Dysoxylin, a limonoid from Dysoxylum richii. Phytochemistry 26: 3309–3311.
Lakshmi, V., K. Pandey, and S.K. Agarwal. 2009. Bioactivity of the compounds in genus Dysoxylum. Acta Ecologica Sinica 29: 30–44.
Pan, L., Y.W. Chin, H.B. Chai, T.N. Ninh, D.D. Soejarto, and A.D. Kinghorn. 2009. Bioactivity-guided isolation of cytotoxic constituents of Brucea javanica collected in Vietnam. Bioorganic and Medicinal Chemistry 17: 2219–2224.
Sichaem, J., S. Surapinit, P. Siripong, S. Khumkratok, J. Jong-aramruang, and S. Tip-pyang. 2010. Two new cytotoxic isomeric indole alkaloids from the roots of Nauclea orientalis. Fitoterapia 81: 830–833.
Singh, Y., and W. Aalbersberg. 1992. Dammarane triterpenoids from Dysoxylum richii. Phytochemistry 31: 4033–4035.
Singh, S., H.S. Garg, and N.M. Khanna. 1976. Dysobinin, a new tetranortriterpene from Dysoxylum binectariferum. Phytochemistry 15: 2001–2002.
Wang, F., and Y.J. Guan. 2012. Cytotoxic nor-dammarane triterpenoids from Dysoxylum hainanense. Fitoterapia 83: 13–17.
Zhang, Y., J.S. Wang, D.D. Wei, X.B. Wang, J. Luo, J.G. Luo, and L.Y. Kong. 2010. Cytotoxic tirucallane C26 triterpenoids from the stem barks of Aphanamixis grandifolia. Phytochemistry 71: 2199–2204.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, P., Liang, G., Gao, X. et al. Three new nor-dammarane triterpenoids from Dysoxylum hainanense with particular cytotoxicity against glioma cell line. Arch. Pharm. Res. 36, 322–326 (2013). https://doi.org/10.1007/s12272-013-0030-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0030-9