Abstract
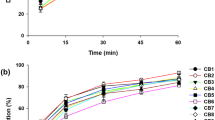
To develop a novel ibuprofen-loaded solid dispersion with enhanced bioavailability, various ibuprofen-loaded solid dispersions were prepared with water, HPMC and poloxamer. The effect of HPMC and poloxamer on aqueous solubility of ibuprofen was investigated. The dissolution and bioavailability of solid dispersion in rats were then evaluated compared to ibuprofen powder. When the amount of carrier increased with a decreased in HPMC/poloxamer ratio, the aqueous solubility of ibuprofen was elevated. The solid dispersion composed of ibuprofen/HPMC/poloxamer at the weight ratio of 10:3:2 improved the drug solubility approximately 4 fold. It gave significantly higher initial plasma concentration, AUC and Cmax of drug than did ibuprofen powder in rats. The solid dispersion improved the bioavailability of drug about 4-fold compared to ibuprofen powder. Thus, this ibuprofen-loaded solid dispersion with water, HPMC and poloxamer was a more effective oral dosage form for improving the bioavailability of poor water-soluble ibuprofen.
Similar content being viewed by others
References
Charoenchaitrakool, M., Dehghani, F., and Foster, N. R., Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-beta-cyclodextrin. Int. J. Pharm., 239, 103–112 (2002).
Chiou, W. L. and Riegelman, S., Pharmaceutical application of solid dispersion systems. J. Pharm. Sci., 73, 1281–1303 (1971).
Choi, H. G., Lee, B. J., Yong, C. S., Rhee, J. D., Han, J. H., Lee, M. K., Park K. M., and Kim, C. K., Terfenadine-β-cyclodextrin inclusion complex with the anti-histaminic activity enhancement. Drug Dev. Ind. Pharm., 27, 857–862 (2001).
Choi, H. G., Oh, Y. K., and Kim, C. K., In-situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int. J. Pharm., 165, 23–32 (1998).
Craig, D. Q. M., The mechanism of drug release from solid dispersion in water-soluble polymers. Int. J. Pharm., 231, 131–144 (2002).
Gerrnhalgh, D. J., Williams, A. C., Timmins, P., and York, P., Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci., 88, 1182–1190 (1999).
Ghorab, M. K. and Adeyeye, M. C., Enhancement of ibuprofen dissolution via wet granulation with beta-cyclodextrin, Pharm. Dev. Technol., 6, 305–314 (2001).
Ghosh, L. K., Ghosh, N. C., Chatterjee, M., and Gupta, B. K., Product development studies on the tablet formulation of ibuprofen to improve bioavailability. Drug Dev. Ind. Pharm., 24, 473–477 (1998).
Glowka, F. K., Stereoselective pharmacokinetics of ibuprofen and its lysinate from suppositories in rabbits. Int. J. Pharm., 199, 159–166 (2000).
Greenhalgh, D. J., Williams, A. C., Timmins, P., York, P., Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci., 88, 1182–1190 (1999).
Kachrimanis, K., Nikolakakis, I., and Malamataris, S., Spherical crystal agglomeration of ibuprofen by the solventchange technique in presence of methacrylic polymers. J. Pharm. Sci., 89, 250–259 (2000).
Khan, G. M. and Jiabi, Z., Preparation, characterization, and dissolution studies of ibuprofen solid dispersions using polyethylene glycol (PEG), talc, and PEG-talc as dispersion carriers. Drug Dev. Ind. Pharm., 24, 455–462 (1998).
Kim, C. K., Choi, J. Y., Yoon, Y. S., Gong, J. P., Choi, H. G., Kong, J. Y., and Lee, B. J., Preparation and evaluation of dry elixir for the enhancement of dissolution rate of poorly water-soluble drugs. Int. J. Pharm., 106, 25–32 (1997).
Leuner, C. and Dressman, J., Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm., 50, 47–60 (2000).
Li, D. X., Oh, Y. K., Lim, S. J., Kim, J. O, Yang, H. J., Sung, J. H., Yong, C. S., and Choi, H. G., Novel gelatin microcapsule with bioavailability enhancement of ibuprofen using spray drying technique. Int. J. Pharm., 355, 277–284 (2008).
Murtha, J. L. and Ando, H. Y., Synthesis of the cholesteryl ester prodrugs cholesteryl ibuprofen and cholesteryl flufenamate and their formulation into phospholipid microemulsions. J. Pharm. Sci., 83, 1222–1228 (1994).
Newa, M., Bhandari, K. H., Li, D. X., Kim, J. O, Yoo, D. S., Kim, J. A., Yoo, B. K., Woo, J. S., Lyoo, W. S., Yong, C. S., and Choi, H. G., Preparation and evaluation of immediate release ibuprofen solid dispersions using polyethylene glycol 4000. Biol. Pharm. Bull., 31, 939–945 (2008).
Newa, M., Bhandari, K. H., Li, D. X., Kwon, T. H., Kim, J. A., Yoo, B. K., Woo, J. S., Lyoo W. S., Yong, C. S., Choi, H. G., Preparation, characterization and in vivo evaluation of ibuprofen binary solid diseprsion with poloxamer 188. Int. J. Pharm., 243, 228–237 (2007).
Passerini, N., Gonzalez-Rodriguez, M. L., Cavallari, C., Rodriguez, L., and Albertini, B., Preparation and characterisation of ibuprofen-poloxamer 188 granules obtained by melt granulation. Eur. J. Pharm. Sci., 15, 71–78 (2002).
Rasenack, N. and Muller, B. W., Dissolution rate enhance-ment by in situ micronization of poorly water-soluble drugs. Pharm. Res., 19, 1894–1900 (2002 a).
Rasenack, N. and Muler, B. W., Properties of ibuprofen crystallized under various conditions: A comparative study. Drug Dev. Ind. Pharm., 28, 1077–1089 (2002 b).
Seo, A., Holm, P., Kristensen, H. G., and Schæfer, T., The preparation of agglomerates containing solid dispersions of diazepam by melt agglomeration in a high shear mixer, Int. J. Pharm., 259, 161–171 (2003).
Society of Toxicology (SOT). Guilding Priciples in the Use of Animals in Toxicology, www.toxicology.org/AI/FA/guidingprinciples.pdf, (1999).
Taylor, L. S. and Zografi, G., Spectroscopic characterization interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res., 14, 1691–1698 (1997).
Yamashita, K., Nakate, T., Okimoto, K., Ohike, A., Tokunaga, Y., Ibuki, R., Higaki, K., and Kimura, T., Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm., 267, 79–91 (2003).
Yong, C. S., Lee, M. K., Park, Y. J., Kong, K. H., Xuan, J. J., Kim, J. H., Kim, J. A., Lyoo, W. S., Han, S. S., Rhee, J. D., Kim, J. O., Yang, C. H., Kim, C. K., and Choi, H. G., Enhanced oral bioavailability of ibuprofen in rats by poloxamer gel using poloxamer 188 and menthol. Drug Dev. Ind. Pharm., 31, 615–622 (2005).
Yong, C. S., Yang, C. H., Rhee, J. D., Lee, B. J., Kim, D. C., Kim, D. D., Kim, C. K., Choi, J. S., and Choi H. C., Enhanced rectal bioavailability of ibuprofen in rats by poloxamer 188 and menthol. Int. J. Pharm., 269, 169–176 (2004).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Park, YJ., Kwon, R., Quan, Q.Z. et al. Development of novel ibuprofen-loaded solid dispersion with improved bioavailability using aqueous solution. Arch. Pharm. Res. 32, 767–772 (2009). https://doi.org/10.1007/s12272-009-1516-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-1516-3