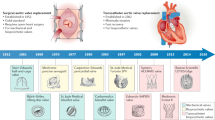
Abstract
Valvular heart disease and congenital heart defects represent a major cause of death around the globe. Although current therapy strategies have rapidly evolved over the decades and are nowadays safe, effective, and applicable to many affected patients, the currently used artificial prostheses are still suboptimal. They do not promote regeneration, physiological remodeling, or growth (particularly important aspects for children) as their native counterparts. This results in the continuous degeneration and subsequent failure of these prostheses which is often associated with an increased morbidity and mortality as well as the need for multiple re-interventions. To overcome this problem, the concept of tissue engineering (TE) has been repeatedly suggested as a potential technology to enable native-like cardiovascular replacements with regenerative and growth capacities, suitable for young adults and children. However, despite promising data from pre-clinical and first clinical pilot trials, the translation and clinical relevance of such TE technologies is still very limited. The reasons that currently limit broad clinical adoption are multifaceted and comprise of scientific, clinical, logistical, technical, and regulatory challenges which need to be overcome. The aim of this review is to provide an overview about the translational problems and challenges in current TE approaches. It further suggests directions and potential solutions on how these issues may be efficiently addressed in the future to accelerate clinical translation. In addition, a particular focus is put on the current regulatory guidelines and the associated challenges for these promising TE technologies.

Similar content being viewed by others
Abbreviations
- EC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- GCP:
-
Good clinical practice
- GLP:
-
Good laboratory practice
- GMP:
-
Good manufacturing practice
- ISO:
-
International Organization for Standardization
- SOP:
-
Standard operating procedure
- TE:
-
Tissue engineering
- TEMP:
-
Tissue engineered medical product
References
Marelli, A. J., Mackie, A. S., Ionescu-Ittu, R., Rahme, E., & Pilote, L. (2007). Congenital heart disease in the general population: changing prevalence and age distribution. Circulation, 115(2), 163–172. doi:10.1161/CIRCULATIONAHA.106.627224.
Nkomo, V. T., Gardin, J. M., Skelton, T. N., Gottdiener, J. S., Scott, C. G., & Enriquez-Sarano, M. Burden of valvular heart diseases: a population-based study. The Lancet, 368(9540), 1005–1011. doi:10.1016/S0140-6736(06)69208-8.
Supino, P. G., Borer, J. S., Preibisz, J., & Bornstein, A. (2006). The epidemiology of valvular heart disease: a growing public health problem. Heart Failure Clinics, 2(4), 379–393. doi:10.1016/j.hfc.2006.09.010.
Cribier, A., Eltchaninoff, H., Bash, A., Borenstein, N., Tron, C., Bauer, F., et al. (2002). Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation, 106(24), 3006–3008.
Fioretta, E. S., Dijkman, P. E., Emmert, M. Y., & Hoerstrup, S. P. (2016). The future of heart valve replacement: recent developments and translational challenges for heart valve tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. doi:10.1002/term.2326.
Schoen, F. J., & Gotlieb, A. I. (2016). Heart valve health, disease, replacement, and repair: a 25-year cardiovascular pathology perspective. Cardiovascular Pathology, 25(4), 341–352. doi:10.1016/j.carpath.2016.05.002.
Alexi-Meskishvilia, V., Ovroutskib, S., Ewertb, P., DaÈhnertb, I., Bergerb, F., Langeb, P. E., et al. (2000). Optimal conduit size for extracardiac Fontan operation. European Journal of Cardio-Thoracic Surgery, 18(690–695).
Yacoub, M. H., & Takkenberg, J. J. (2005). Will heart valve tissue engineering change the world? Nature Clinical Practice. Cardiovascular Medicine, 2(2), 60–61. doi:10.1038/ncpcardio0112.
Mendelson, K., & Schoen, F. J. (2006). Heart valve tissue engineering: concepts, approaches, progress, and challenges. Annals of Biomedical Engineering, 34(12), 1799–1819. doi:10.1007/s10439-006-9163-z.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926.
Dohmen, P. M., Lembcke, A., Hotz, H., Kivelitz, D., & Konertz, W. F. (2002). Ross operation with a tissue-engineered heart valve. The Annals of Thoracic Surgery, 74(5), 1438–1442.
Dohmen, P. M., Lembcke, A., Holinski, S., Pruss, A., & Konertz, W. (2011). Ten years of clinical results with a tissue-engineered pulmonary valve. The Annals of Thoracic Surgery, 92(4), 1308–1314. doi:10.1016/j.athoracsur.2011.06.009.
Cebotari, S., Lichtenberg, A., Tudorache, I., Hilfiker, A., Mertsching, H., Leyh, R., et al. (2006). Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation, 114(1 Suppl), I132–I137. doi:10.1161/CIRCULATIONAHA.105.001065.
Dohmen, P. M., Lembcke, A., Holinski, S., Kivelitz, D., Braun, J. P., Pruss, A., et al. (2007). Mid-term clinical results using a tissue-engineered pulmonary valve to reconstruct the right ventricular outflow tract during the Ross procedure. The Annals of Thoracic Surgery, 84(3), 729–736. doi:10.1016/j.athoracsur.2007.04.072.
Naito, Y., Imai, Y., Shin’oka, T., Kashiwagi, J., Aoki, M., Watanabe, M., et al. (2003). Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. The Journal of Thoracic and Cardiovascular Surgery, 125(2), 419–420. doi:10.1067/mtc.2003.134.
Shinoka, T., & Breuer, C. (2008). Tissue-engineered blood vessels in pediatric cardiac surgery. Yale Journal of Biology and Medicine, 81, 161–166.
Bouten, C. V., Dankers, P. Y., Driessen-Mol, A., Pedron, S., Brizard, A. M., & Baaijens, F. P. (2011). Substrates for cardiovascular tissue engineering. Advanced Drug Delivery Reviews, 63(4–5), 221–241. doi:10.1016/j.addr.2011.01.007.
Fioretta, E. S., Fledderus, J. O., Burakowska-Meise, E. A., Baaijens, F. P., Verhaar, M. C., & Bouten, C. V. (2012). Polymer-based scaffold designs for in situ vascular tissue engineering: controlling recruitment and differentiation behavior of endothelial colony forming cells. Macromolecular Bioscience, 12(5), 577–590. doi:10.1002/mabi.201100315.
Dijkman, P. E., Fioretta, E. S., Frese, L., Pasqualini, F. S., & Hoerstrup, S. P. (2016). Heart valve replacements with regenerative capacity. Transfusion Medicine and Hemotherapy, 43(4), 282–290. doi:10.1159/000448181.
Cheung, D. Y., Duan, B., & Butcher, J. T. (2015). Current progress in tissue engineering of heart valves: multiscale problems, multiscale solutions. Expert opinion on biological therapy. [early online].
Jana, S., Tefft, B. J., Spoon, D. B., & Simari, R. D. (2014). Scaffolds for tissue engineering of cardiac valves. Acta Biomaterialia, 10(7), 2877–2893. doi:10.1016/j.actbio.2014.03.014.
Kurobe, H., Maxfield, M. W., Breuer, C. K., & Shinoka, T. (2012). Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med, 1(7), 566–571. doi:10.5966/sctm.2012-0044.
Patterson, J. T., Gilliland, T., Maxfield, M. W., Church, S., Naito, Y., Shinoka, T., et al. (2012). Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regenerative Medicine, 7(3), 409–419. doi:10.2217/rme.12.12.
Fioretta, E. S., Fledderus, J. O., Baaijens, F. P., & Bouten, C. V. (2012). Influence of substrate stiffness on circulating progenitor cell fate. Journal of Biomechanics, 45(5), 736–744. doi:10.1016/j.jbiomech.2011.11.013.
Tudorache, I., Horke, A., Cebotari, S., Sarikouch, S., Boethig, D., Breymann, T., et al. (2016). Decellularized aortic homografts for aortic valve and aorta ascendens replacement. European Journal of Cardio-Thoracic Surgery, 50(1), 89–97. doi:10.1093/ejcts/ezw013.
Sarikouch, S., Horke, A., Tudorache, I., Beerbaum, P., Westhoff-Bleck, M., Boethig, D., et al. (2016). Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. European Journal of Cardio-Thoracic Surgery. doi:10.1093/ejcts/ezw050.
Neumann, A., Cebotari, S., Tudorache, I., Haverich, A., & Sarikouch, S. (2013). Heart valve engineering: decellularized allograft matrices in clinical practice. Biomed Tech (Berl), 58(5), 453–456. doi:10.1515/bmt-2012-0115.
Cebotari, S., Tudorache, I., Ciubotaru, A., Boethig, D., Sarikouch, S., Goerler, A., et al. (2011). Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation, 124(11 Suppl), S115–S123. doi:10.1161/CIRCULATIONAHA.110.012161.
da Costa, F. D. A., Costa, A. C. B. A., Prestes, R., Domanski, A. C., Balbi, E. M., Ferreira, A. D. A., et al. (2010). The early and midterm function of decellularized aortic valve allografts. The Annals of Thoracic Surgery, 90(6), 1854–1860. doi:10.1016/j.athoracsur.2010.08.022.
Hoerstrup, S. P., Sodian, R., Daebritz, S., Wang, J., Bacha, E. A., Martin, D. P., et al. (2000). Functional living trileaflet heart valves grown in vitro. Circulation, 102(19 Suppl 3), III44–III49.
Hoerstrup, S. P., Cummings Mrcs, I., Lachat, M., Schoen, F. J., Jenni, R., Leschka, S., et al. (2006). Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation, 114(1 Suppl), I159–I166. doi:10.1161/CIRCULATIONAHA.105.001172.
Shinoka, T., Ma, P. X., Shum-Tim, D., Breuer, C. K., Cusick, R. A., Zund, G., et al. (1996). Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model. Circulation, 94(9), II164–II168.
Weber, B., Scherman, J., Emmert, M. Y., Gruenenfelder, J., Verbeek, R., Bracher, M., et al. (2011). Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. European Heart Journal, 32(22), 2830–2840. doi:10.1093/eurheartj/ehr059.
Driessen-Mol, A., Emmert, M. Y., Dijkman, P. E., Frese, L., Sanders, B., Weber, B., et al. (2014). Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep. Journal of the American College of Cardiology, 63(13), 1320–1329. doi:10.1016/j.jacc.2013.09.082.
Syedain, Z., Reimer, J., Schmidt, J., Lahti, M., Berry, J., Bianco, R., et al. (2015). 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials, 73, 175–184. doi:10.1016/j.biomaterials.2015.09.016.
Syedain, Z., Reimer, J., Lahti, M., Berry, J., Johnson, S., & Tranquillo, R. T. (2016). Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nature Communications, 7, 12951. doi:10.1038/ncomms12951.
Sievers, H.-H., Stierle, U., Schmidtke, C., & Bechtel, M. (2003). Decellularized pulmonary homograft (SynerGraft) for reconstruction of the right ventricular outflow tract: first clinical experience. [journal article]. Zeitschrift für Kardiologie, 92(1), 53–59. doi:10.1007/s00392-003-0883-x.
Hibino, N., McConnell, P., Shinoka, T., Malik, M., & Galantowicz, M. (2015). Preliminary experience in the use of an extracellular matrix (CorMatrix) as a tube graft: word of caution. Seminars in thoracic and cardiovascular surgery, doi:10.1053/j.semtcvs.2015.08.008.
Iop, L., & Gerosa, G. (2015). Guided tissue regeneration in heart valve replacement: from preclinical research to first-in-human trials. BioMed Research International, 2015, 432901. doi:10.1155/2015/432901.
Salmikangas, P., Schuessler-Lenz, M., Ruiz, S., Celis, P., Reischl, I., Menezes-Ferreira, M., et al. (2015). Marketing regulatory oversight of advanced therapy medicinal products (ATMPs) in Europe: the EMA/CAT perspective. Advances in Experimental Medicine and Biology, 871, 103–130. doi:10.1007/978-3-319-18618-4_6.
Yano, K., Watanabe, N., Tsuyuki, K., Ikawa, T., Kasanuki, H., & Yamato, M. (2015). Regulatory approval for autologous human cells and tissue products in the United States, the European Union, and Japan. Regenerative Therapy, 1, 45–56. doi:10.1016/j.reth.2014.10.001.
Sanzenbacher, R., Dwenger, A., Schuessler-Lenz, M., Cichutek, K., & Flory, E. (2007). European regulation tackles tissue engineering. Nat Biotech, 25(10), 1089–1091.
Commission Directive 2009/120/EC of 14 September 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use as regards advanced therapy medicinal products (2009). Official Journal of the European Union, 242, 3).
Lee, M. H., Arcidiacono, J. A., Bilek, A. M., Wille, J. J., Hamill, C. A., Wonnacott, K. M., et al. (2010). Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Engineering. Part B, Reviews, 16(1), 41–54.
Lu, L., Arbit, H. M., Herrick, J. L., Segovis, S. G., Maran, A., & Yaszemski, M. J. (2015). Tissue engineered constructs: perspectives on clinical translation. Annals of Biomedical Engineering, 43(3), 796–804. doi:10.1007/s10439-015-1280-0.
Law, E. U. (2012). Proposal for a regulation of the European Parliament and of the council on medical devices. EUR-Lex, 52012PC0542, http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52012PC50542.
Lichtenberg, A., Tudorache, I., Cebotari, S., Suprunov, M., Tudorache, G., Goerler, H., et al. (2006). Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation, 114(1 Suppl), I559–I565. doi:10.1161/CIRCULATIONAHA.105.001206.
Baraki, H., Tudorache, I., Braun, M., Hoffler, K., Gorler, A., Lichtenberg, A., et al. (2009). Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials, 30(31), 6240–6246. doi:10.1016/j.biomaterials.2009.07.068.
Iop, L., Bonetti, A., Naso, F., Rizzo, S., Cagnin, S., Bianco, R., et al. (2014). Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PloS One, 9(6), e99593. doi:10.1371/journal.pone.0099593.
Row, S., Peng, H., Schlaich, E. M., Koenigsknecht, C., Andreadis, S. T., & Swartz, D. D. (2015). Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: the role of cells in the vascular wall. Biomaterials, 50, 115–126. doi:10.1016/j.biomaterials.2015.01.045.
Koobatian, M. T., Row, S., Smith Jr., R. J., Koenigsknecht, C., Andreadis, S. T., & Swartz, D. D. (2016). Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials, 76, 344–358. doi:10.1016/j.biomaterials.2015.10.020.
Simon, P., Kasimir, M. T., Seebacher, G., Weigel, G., Allrich, R., Salzer-Muhar, U., et al. (2003). Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. European Journal of Cardio-Thoracic Surgery, 23(6), 1002–1006.
Voges, I., Brasen, J. H., Entenmann, A., Scheid, M., Scheewe, J., Fischer, G., et al. (2013). Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. European Journal of Cardio-Thoracic Surgery, 44(4), e272–e279. doi:10.1093/ejcts/ezt328.
Ruffer, A., Purbojo, A., Cicha, I., Glockler, M., Potapov, S., Dittrich, S., et al. (2010). Early failure of xenogenous de-cellularised pulmonary valve conduits—a word of caution! European Journal of Cardio-Thoracic Surgery, 38(1), 78–85. doi:10.1016/j.ejcts.2010.01.044.
Woo, J. S., Fishbein, M. C., & Reemtsen, B. (2015). Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovascular Pathology. doi:10.1016/j.carpath.2015.08.007.
Watanabe, M., Shin’oka, T., Tohyama, S., Hibino, N., Konuma, T., Matsumura, G., et al. (2001). Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Engineering, 7(4), 429–439.
Hoerstrup, S. P., Kadner, A., Melnitchouk, S., Trojan, A., Eid, K., Tracy, J., et al. (2002). Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation, 106(suppl I), I-143–I-150. doi:10.1161/01.cir.0000032872.55215.05.
Frese, L., Sanders, B., Beer, G. M., Weber, B., Driessen-Mol, A., Baaijens, F. P. T., et al. (2015). Adipose derived tissue engineered heart valve. Journal of Tissue Science & Engineering, 06(03). doi:10.4172/2157-7552.1000156.
Sodian, R., Schaefermeier, P., Begg-Zips, S., Kuebler, W. M., Shakibaei, M., & Daebritz, S. (2010). Use of human umbilical cord blood-derived progenitor cells for tissue-engineered heart valves. Ann.Thorac.Surg., 89(3), 819–828.
Bayon, Y., Vertes, A. A., Ronfard, V., Egloff, M., Snykers, S., Salinas, G. F., et al. (2014). Translating cell-based regenerative medicines from research to successful products: challenges and solutions. Tissue Engineering. Part B, Reviews, 20(4), 246–256. doi:10.1089/ten.TEB.2013.0727.
Fioretta, E. S., Simonet, M., Smits, A. I., Baaijens, F. P., & Bouten, C. V. (2014). Differential response of endothelial and endothelial colony forming cells on electrospun scaffolds with distinct microfiber diameters. Biomacromolecules, 15(3), 821–829. doi:10.1021/bm4016418.
Nisbet, D. R., Forsythe, J. S., Shen, W., Finkelstein, D. I., & Horne, M. K. (2009). Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. Journal of Biomaterials Applications, 24(1), 7–29.
Jana, S., Tranquillo, R. T., & Lerman, A. (2016). Cells for tissue engineering of cardiac valves. Journal of Tissue Engineering and Regenerative Medicine, 10(10), 804–824. doi:10.1002/term.2010.
da Costa, F. D., Dohmen, P. M., Duarte, D., von Glenn, C., Lopes, S. V., Filho, H. H., et al. (2005). Immunological and echocardiographic evaluation of decellularized versus cryopreserved allografts during the Ross operation. European Journal of Cardio-Thoracic Surgery, 27(4), 572–578. doi:10.1016/j.ejcts.2004.12.057.
Dijkman, P. E., Driessen-Mol, A., Frese, L., Hoerstrup, S. P., & Baaijens, F. P. (2012). Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials, 33(18), 4545–4554. doi:10.1016/j.biomaterials.2012.03.015.
Syedain, Z. H., Meier, L. A., Reimer, J. M., & Tranquillo, R. T. (2013). Tubular heart valves from decellularized engineered tissue. Annals of Biomedical Engineering, 41(12), 2645–2654. doi:10.1007/s10439-013-0872-9.
Melchiorri, A. J., Hibino, N., Yi, T., Lee, Y. U., Sugiura, T., Tara, S., et al. (2015). Contrasting biofunctionalization strategies for the enhanced endothelialization of biodegradable vascular grafts. Biomacromolecules, 16(2), 437–446. doi:10.1021/bm501853s.
Tara, S., Kurobe, H., Maxfield, M. W., Rocco, K. A., Yi, T., Naito, Y., et al. (2015). Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. Journal of Vascular Surgery, 62(3), 734–743. doi:10.1016/j.jvs.2014.03.011.
Talacua, H., Smits, A. I., Muylaert, D. E., van Rijswijk, J. W., Vink, A., Verhaar, M. C., et al. (2015). In situ tissue engineering of functional small-diameter blood vessels by host circulating cells only. Tissue Engineering. Part A, 21(19–20), 2583–2594. doi:10.1089/ten.TEA.2015.0066.
Ekdahl, K. N., Lambris, J. D., Elwing, H., Ricklin, D., Nilsson, P. H., Teramura, Y., et al. (2011). Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Advanced Drug Delivery Reviews, 63(12), 1042–1050. doi:10.1016/j.addr.2011.06.012.
Smyth, J. V., Welch, M., Carr, H. M., Dodd, P. D., Eisenberg, P. R., & Walker, M. G. (1995). Fibrinolysis profiles and platelet activation after endothelial cell seeding of prosthetic vascular grafts. Annals of Vascular Surgery, 9(6), 542–546. doi:10.1007/BF02018827.
Laube, H. R., Duwe, J., Rutsch, W., & Konertz, W. (2000). Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. The Journal of Thoracic and Cardiovascular Surgery, 120(1), 134–141. doi:10.1067/mtc.2000.106327.
Zilla, P., Fasol, R., Deutsch, M., Fischlein, T., Minar, E., Hammerle, A., et al. (1987). Endothelial cell seeding of polytetrafluoroethylene vascular grafts in humans: a preliminary report. Journal of Vascular Surgery, 6(6), 535–541. doi:10.1016/0741-5214(87)90266-7.
Herring, M. B. (1991). Endothelial cell seeding. Journal of Vascular Surgery, 13(5), 731–732. doi:10.1016/0741-5214(91)90365-2.
Shi, Z., Neoh, K. G., & Kang, E. T. (2013). In vitro endothelialization of cobalt chromium alloys with micro/nanostructures using adipose-derived stem cells. Journal of Materials Science: Materials in Medicine, 24(4), 1067–1077. doi:10.1007/s10856-013-4868-7.
Kim, Y., & Liu, J. C. (2016). Protein-engineered microenvironments can promote endothelial differentiation of human mesenchymal stem cells in the absence of exogenous growth factors. Biomaterials Science, 4(12), 1761–1772. doi:10.1039/C6BM00472E.
Melchiorri, A. J., Hibino, N., & Fisher, J. P. (2013). Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts. Tissue Engineering. Part B, Reviews, 19(4), 292–307. doi:10.1089/ten.TEB.2012.0577.
Lin, Q., Ding, X., Qiu, F., Song, X., Fu, G., & Ji, J. (2010). In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayer. Biomaterials, 31(14), 4017–4025. doi:10.1016/j.biomaterials.2010.01.092.
Rotmans, J. I., Heyligers, J. M., Verhagen, H. J., Velema, E., Nagtegaal, M. M., de Kleijn, D. P., et al. (2005). In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation, 112(1), 12–18. doi:10.1161/CIRCULATIONAHA.104.504407.
Lu, S., Zhang, P., Sun, X., Gong, F., Yang, S., Shen, L., et al. (2013). Synthetic ePTFE grafts coated with an anti-CD133 antibody-functionalized heparin/collagen multilayer with rapid in vivo endothelialization properties. ACS Applied Materials & Interfaces, 5(15), 7360–7369. doi:10.1021/am401706w.
Jordan, J. E., Williams, J. K., Lee, S. J., Raghavan, D., Atala, A., & Yoo, J. J. (2012). Bioengineered self-seeding heart valves. The Journal of Thoracic and Cardiovascular Surgery, 143(1), 201–208. doi:10.1016/j.jtcvs.2011.10.005.
Ravi, S., Qu, Z., & Chaikof, E. L. (2009). Polymeric materials for tissue engineering of arterial substitutes. Vascular, 17(Supplement 1), S45-S54, doi:10.2310/6670.2008.00084.
Zheng, W., Wang, Z., Song, L., Zhao, Q., Zhang, J., Li, D., et al. (2012). Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials, 33(10), 2880–2891. doi:10.1016/j.biomaterials.2011.12.047.
Caiado, F., Carvalho, T., Silva, F., Castro, C., Clode, N., Dye, J. F., et al. (2011). The role of fibrin E on the modulation of endothelial progenitors adhesion, differentiation and angiogenic growth factor production and the promotion of wound healing. Biomaterials, 32(29), 7096–7105. doi:10.1016/j.biomaterials.2011.06.022.
Rodenberg, E. J., & Pavalko, F. M. (2007). Peptides derived from fibronectin type III connecting segments promote endothelial cell adhesion but not platelet adhesion: implications in tissue-engineered vascular grafts. Tissue Engineering, 13(11), 2653–2666. doi:10.1089/ten.2007.0037.
Jun, H.-W., & West, J. L. (2005). Modification of polyurethaneurea with PEG and YIGSR peptide to enhance endothelialization without platelet adhesion. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 72B(1), 131–139. doi:10.1002/jbm.b.30135.
Aubin, H., Mas-Moruno, C., Iijima, M., Schutterle, N., Steinbrink, M., Assmann, A., et al. (2016). Customized interface biofunctionalization of decellularized extracellular matrix: toward enhanced endothelialization. Tissue Engineering. Part C, Methods, 22(5), 496–508. doi:10.1089/ten.TEC.2015.0556.
Liu, P., Zhao, Y., Yan, Y., Hu, Y., Yang, W., & Cai, K. (2015). Construction of extracellular microenvironment to improve surface endothelialization of NiTi alloy substrate. Mater Sci Eng C Mater Biol Appl, 55, 1–7. doi:10.1016/j.msec.2015.05.047.
Smith Jr., R. J., Koobatian, M. T., Shahini, A., Swartz, D. D., & Andreadis, S. T. (2015). Capture of endothelial cells under flow using immobilized vascular endothelial growth factor. Biomaterials, 51, 303–312. doi:10.1016/j.biomaterials.2015.02.025.
Wang, H., Leinwand, L. A., & Anseth, K. S. (2014). Cardiac valve cells and their microenvironment—insights from in vitro studies. Nature Reviews. Cardiology, 11(12), 715–727. doi:10.1038/nrcardio.2014.162.
Yin, Y., Zhao, X., Fang, Y., Yu, S., Zhao, J., Song, M., et al. (2010). SDF-1α involved in mobilization and recruitment of endothelial progenitor cells after arterial injury in mice. Cardiovascular Pathology, 19(4), 218–227. doi:10.1016/j.carpath.2009.04.002.
Liu, T., Liu, S., Zhang, K., Chen, J., & Huang, N. (2014). Endothelialization of implanted cardiovascular biomaterial surfaces: the development from in vitro to in vivo. Journal of Biomedical Materials Research. Part A, 102(10), 3754–3772. doi:10.1002/jbm.a.35025.
Emmert, M. Y., & Hoerstrup, S. P. (2016). Tissue engineered heart valves: moving towards clinical translation. Expert Review of Medical Devices, 13(5), 417–419. doi:10.1586/17434440.2016.1171709.
Beebe, D. J., Ingber, D. E., & den Toonder, J. (2013). Organs on chips 2013. Lab on a Chip, 13(18), 3447–3448. doi:10.1039/c3lc90080k.
Ram-Liebig, G., Bednarz, J., Stuerzebecher, B., Fahlenkamp, D., Barbagli, G., Romano, G., et al. (2015). Regulatory challenges for autologous tissue engineered products on their way from bench to bedside in Europe. Advanced Drug Delivery Reviews, 82-83, 181–191. doi:10.1016/j.addr.2014.11.009.
Hurtado-Aguilar, L. G., Mulderrig, S., Moreira, R., Hatam, N., Spillner, J., Schmitz-Rode, T., et al. (2016). Ultrasound for in vitro noninvasive, real time monitoring and evaluation of tissue-engineered heart valves. Tissue Engineering. Part C, Methods. doi:10.1089/ten.TEC.2016.0300.
Ozaki, S., Herijgers, P., & Flameng, W. (2004). A new model to test the calcification characteristics of bioprosthetic heart valves. Annals of Thoracic and Cardiovascular Surgery, 10, 23–28.
Taramasso, M., Emmert, M. Y., Reser, D., Guidotti, A., Cesarovic, N., Campagnol, M., et al. (2015). Pre-clinical in vitro and in vivo models for heart valve therapies. Journal of Cardiovascular Translational Research, 8(5), 319–327. doi:10.1007/s12265-015-9631-7.
Yuan, S. M., Mishaly, D., Shinfeld, A., & Raanani, E. (2008). Right ventricular outflow tract reconstruction: valved conduit of choice and clinical outcomes. Journal of Cardiovascular Medicine, 9, 327–337.
Bayon, Y., Vertes, A. A., Ronfard, V., Culme-Seymour, E., Mason, C., Stroemer, P., et al. (2015). Turning regenerative medicine breakthrough ideas and innovations into commercial products. Tissue Engineering. Part B, Reviews, 21(6), 560–571. doi:10.1089/ten.TEB.2015.0068.
de Wilde, S., Guchelaar, H. J., Herberts, C., Lowdell, M., Hildebrandt, M., Zandvliet, M., et al. (2016). Development of cell therapy medicinal products by academic institutes. Drug Discovery Today, 21(8), 1206–1212. doi:10.1016/j.drudis.2016.04.016.
Acknowledgments
The authors have received funding support from the European FP 7 Framework Programme under grant agreement no. 604514 (ImaValve).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Associate Editor Adrian Chester oversaw the review of this article
Maximilian Y. Emmert and Emanuela S. Fioretta contributed equally.
Rights and permissions
About this article
Cite this article
Emmert, M.Y., Fioretta, E.S. & Hoerstrup, S.P. Translational Challenges in Cardiovascular Tissue Engineering. J. of Cardiovasc. Trans. Res. 10, 139–149 (2017). https://doi.org/10.1007/s12265-017-9728-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-017-9728-2