Abstract
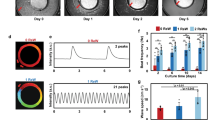
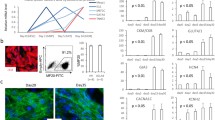
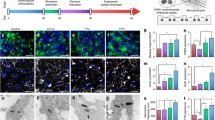
While human embryonic stem cells (hESCs) can differentiate into functional cardiomyocytes, their immature phenotypes limit their therapeutic application for myocardial regeneration. We sought to determine whether electrical stimulation could enhance the differentiation and maturation of hESC-derived cardiomyocytes. Cardiac differentiation was induced in a HES3 hESC line via embryoid bodies formation treated with a p38 MAP kinase inhibitor. Detailed molecular and functional analysis were performed in those hESC-derived cardiomyocytes cultured for 4 days in the absence or presence of electrical field stimulation (6.6 V/cm, 1 Hz, and 2 ms pulses) using an eight-channel C-Pace stimulator (Ion-Optics Co., MA). Upon electrical stimulation, quantitative polymerase chain reaction demonstrated significant upregulation of cardiac-specific gene expression including HCN1, MLC2V, SCN5A, SERCA, Kv4.3, and GATA4; immunostaining and flow cytometry analysis revealed cellular elongation and an increased proportion of troponin-T positive cells (6.3 ± 1.2 % vs. 15.8 ± 2.1 %; n = 3, P < 0.01). Electrophysiological studies showed an increase in the proportion of ventricular-like hESC-derived cardiomyocytes (48 vs. 29 %, P < 0.05) with lengthening of their action potential duration at 90 % repolarization (387.7 ± 35.35; n = 11 vs. 291.8 ± 20.82; n = 10, P < 0.05) and 50 % repolarization (313.9 ± 27.94; n = 11 vs. 234.0 ± 16.10; n = 10, P < 0.05) after electrical stimulation. Nonetheless, the membrane diastolic potentials and action potential upstrokes of different hESC-derived cardiomyocyte phenotypes, and the overall beating rate remained unchanged (all P > 0.05). Fluorescence confocal imaging revealed that electrical stimulation significantly increased both spontaneous and caffeine-induced calcium flux in the hESC-derived cardiomyocytes (approximately 1.6-fold for both cases; P < 0.01). In conclusion, electrical field stimulation increased the expression of cardiac-specific genes and the yield of differentiation, promoted ventricular-like phenotypes, and improved the calcium handling of hESC-derived cardiomyocytes.






Similar content being viewed by others
References
Senyo, S. E., Steinhauser, M. L., Pizzimenti, C. L., Yang, V. K., Cai, L., Wang, M., et al. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature, 493(7432), 433–436.
Siu, C. W., & Tse, H. F. (2012). Cardiac regeneration: messages from CADUCEUS. Lancet, 379(9819), 870–871.
Liao, S. Y., Liu, Y., Siu, C. W., Zhang, Y., Lai, W. H., Au, K. W., et al. (2010). Proarrhythmic risk of embryonic stem cell-derived cardiomyocyte transplantation in infarcted myocardium. Heart Rhythm, 7(12), 1852–1859.
Liao, S. Y., Tse, H. F., Chan, Y. C., Mei-Chu Yip, P., Zhang, Y., Liu, Y., et al. (2013). Overexpression of Kir2.1 channel in embryonic stem cell-derived cardiomyocytes attenuates posttransplantation proarrhythmic risk in myocardial infarction. Heart Rhythm, 10(2), 273–282.
Ng, K. M., Lee, Y. K., Chan, Y. C., Lai, W. H., Fung, M. L., Li, R. A., et al. (2010). Exogenous expression of HIF-1 alpha promotes cardiac differentiation of embryonic stem cells. Journal of Molecular and Cellular Cardiology, 48(6), 1129–1137.
Ng, K. M., Chan, Y. C., Lee, Y. K., Lai, W. H., Au, K. W., Fung, M. L., et al. (2011). Cobalt chloride pretreatment promotes cardiac differentiation of human embryonic stem cells under atmospheric oxygen level. Cellular Reprogramming, 13(6), 527–537.
Cameron, I. L., Hardman, W. E., Winters, W. D., Zimmerman, S., & Zimmerman, A. M. (1993). Environmental magnetic fields: influences on early embryogenesis. Journal of Cellular Biochemistry, 51(4), 417–425.
Robinson, K. R. (1985). The responses of cells to electrical fields: a review. The Journal of Cell Biology, 101(6), 2023–2027.
Chen, M. Q., Xie, X., Hollis Whittington, R., Kovacs, G. T., Wu, J. C., & Giovangrandi, L. (2008). Cardiac differentiation of embryonic stem cells with point-source electrical stimulation. Conference Proceedings, IEEE Engineering in Medicine and Biology Society, 2008, 1729–1732.
Sauer, H., Rahimi, G., Hescheler, J., & Wartenberg, M. (1999). Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. Journal of Cellular Biochemistry, 75(4), 710–723.
Serena, E., Figallo, E., Tandon, N., Cannizzaro, C., Gerecht, S., Elvassore, N., et al. (2009). Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Experimental Cell Research, 315(20), 3611–3619.
Choo, A., Padmanabhan, J., Chin, A., Fong, W. J., & Oh, S. K. (2006). Immortalized feeders for the scale-up of human embryonic stem cells in feeder and feeder-free conditions. Journal of Biotechnology, 122(1), 130–141.
Ting, S., Lecina, M., Reuveny, S., & Oh, S. (2012). Differentiation of human embryonic stem cells to cardiomyocytes on microcarrier cultures. Current Protocols in Stem Cell Biology, Chapter 1, Unit1D 7.
Lecina, M., Ting, S., Choo, A., Reuveny, S., & Oh, S. (2010). Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Engineering. Part C, Methods, 16(6), 1609–1619.
Chan, Y. C., Siu, C. W., Lau, Y. M., Lau, C. P., Li, R. A., & Tse, H. F. (2009). Synergistic effects of inward rectifier (I) and pacemaker (I) currents on the induction of bioengineered cardiac automaticity. Journal of Cardiovascular Electrophysiology, 20(9), 1048–1054.
Tandon, N., Cannizzaro, C., Chao, P. H., Maidhof, R., Marsano, A., Au, H. T., et al. (2009). Electrical stimulation systems for cardiac tissue engineering. Nature Protocols, 4(2), 155–173.
Pu, W. T., Ishiwata, T., Juraszek, A. L., Ma, Q., & Izumo, S. (2004). GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Biology, 275(1), 235–244.
Zeisberg, E. M., Ma, Q., Juraszek, A. L., Moses, K., Schwartz, R. J., Izumo, S., et al. (2005). Morphogenesis of the right ventricle requires myocardial expression of Gata4. Journal of Clinical Investigation, 115(6), 1522–1531.
Charpentier, F., Merot, J., Loussouarn, G., & Baro, I. (2010). Delayed rectifier K(+) currents and cardiac repolarization. Journal of Molecular and Cellular Cardiology, 48(1), 37–44.
Ravens, U., & Wettwer, E. (1998). Electrophysiological aspects of changes in heart rate. Basic Research in Cardiology, 93(Suppl 1), 60–65.
Qu, Z., & Chung, D. (2012). Mechanisms and determinants of ultralong action potential duration and slow rate-dependence in cardiac myocytes. PLoS One, 7(8), e43587.
Genovese, J. A., Spadaccio, C., Chachques, E., Schussler, O., Carpentier, A., Chachques, J. C., et al. (2009). Cardiac pre-differentiation of human mesenchymal stem cells by electrostimulation. Frontiers in Bioscience, 14, 2996–3002.
Genovese, J. A., Spadaccio, C., Langer, J., Habe, J., Jackson, J., & Patel, A. N. (2008). Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts. Biochemical and Biophysical Research Communications, 370(3), 450–455.
Gamry Instruments. (2005). Electrochemical impedance spectroscopy theory: a primer.
Setsukinai, K., Urano, Y., Kakinuma, K., Majima, H. J., & Nagano, T. (2003). Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. Journal of Biological Chemistry, 278(5), 3170–3175.
Sauer, H., & Wartenberg, M. (2005). Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxidants and Redox Signaling, 7(11–12), 1423–1434.
Puceat, M., Travo, P., Quinn, M. T., & Fort, P. (2003). A dual role of the GTPase Rac in cardiac differentiation of stem cells. Molecular Biology of the Cell, 14(7), 2781–2792.
Puceat, M. (2005). Role of Rac-GTPase and reactive oxygen species in cardiac differentiation of stem cells. Antioxidants and Redox Signaling, 7(11–12), 1435–1439.
Cannizzaro, C., Tandon, N., Figallo, E., Park, H., Gerecht, S., Radisic, M., et al. (2007). Practical aspects of cardiac tissue engineering with electrical stimulation. Methods in Molecular Medicine, 140, 291–307.
Huang, J. Z. C., Zhang, W., & Zhou, X. (1997). Application of a platinum dual-disk microelectrode to measurement of the electron transfer number of dioxygen reduction. Journal of Electroanalytical Chemistry, 433, 33–39.
Li, J., Stouffs, M., Serrander, L., Banfi, B., Bettiol, E., Charnay, Y., et al. (2006). The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Molecular Biology of the Cell, 17(9), 3978–3988.
Radisic, M., Park, H., Shing, H., Consi, T., Schoen, F. J., Langer, R., et al. (2004). Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America, 101(52), 18129–18134.
Satin, J., Itzhaki, I., Rapoport, S., Schroder, E. A., Izu, L., Arbel, G., et al. (2008). Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells, 26(8), 1961–1972.
Burridge, P. W., Keller, G., Gold, J. D., & Wu, J. C. (2012). Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell, 10(1), 16–28.
Loeb, G. E., Zamin, C. J., Schulman, J. H., & Troyk, P. R. (1991). Injectable microstimulator for functional electrical stimulation. Medical & Biological Engineering & Computing, 29(6), NS13–19.
Tandon, N., Goh, B., Marsano, A., Chao, P. H., Montouri-Sorrentino, C., Gimble, J., et al. (2009). Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation. Conference Proceedings, IEEE Engineering in Medicine and Biology Society, 2009, 6517–6521.
Levin, M. (2003). Motor protein control of ion flux is an early step in embryonic left-right asymmetry. Bioessays, 25(10), 1002–1010.
Donaldson, N. D., & Donaldson, P. E. (1986). When are actively balanced biphasic (‘lilly’) stimulating pulses necessary in a neurological prosthesis? I. Historical background; Pt resting potential; Q studies. Medical & Biological Engineering & Computing, 24, 41–49.
Acknowledgments
This study was supported by Hong Kong Research Grant Council (HKU 8/CRF/09, HKU 8/CRF/10, HKU 780110 M to H.F.T), Theme-based Research Scheme (T12-705/11 to C.W.S and H.F.T), and CRCG Small Project Funding of University of Hong Kong (Y.C.C); Agency for Science Technology and Research (S.T. and S.K.O).
Disclosure Statement
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer L. Hall oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Chan, YC., Ting, S., Lee, YK. et al. Electrical Stimulation Promotes Maturation of Cardiomyocytes Derived from Human Embryonic Stem Cells. J. of Cardiovasc. Trans. Res. 6, 989–999 (2013). https://doi.org/10.1007/s12265-013-9510-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9510-z