Abstract
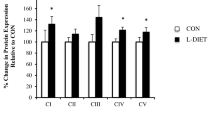
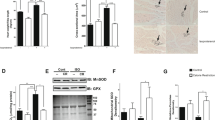
Obesity is associated with increased diastolic stiffness and myocardial steatosis and dysfunction. The impact of aging on the protective effects of caloric restriction (CR) is not clear. We studied 2-month (younger) and 6–7-month (older)-old ob/ob mice and age-matched C57BL/6J controls (WT). Ob/ob mice were assigned to diet ad libitum or CR for 4 weeks. We performed echocardiograms, myocardial triglyceride assays, Oil Red O staining, and measured free fatty acids, superoxide, NOS activity, ceramide levels, and Western blots. In younger mice, CR restored diastolic function, reversed myocardial steatosis, and upregulated Akt phosphorylation. None of these changes was observed in the older mice; however, CR decreased oxidative stress and normalized NOS activity in these animals. Interestingly, myocardial steatosis was not associated with increased ceramide, but CR altered the composition of ceramides. In this model of obesity, aging attenuates the benefits of CR on myocardial structure and function.






Similar content being viewed by others
References
Cepeda-Valery, B., Pressman, G. S., Figueredo, V. M., & Romero-Corral, A. (2011). Impact of obesity on total and cardiovascular mortality—fat or fiction? Nature Reviews Cardiology, 8, 233–237.
Barouch, L. A., Berkowitz, D. E., Harrison, R. W., O'Donnell, C. P., & Hare, J. M. (2003). Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation, 108, 754–759.
Mazumder, P. K., O'Neill, B. T., Roberts, M. W., et al. (2004). Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes, 53, 2366–2374.
Abel, E. D., Litwin, S. E., & Sweeney, G. (2008). Cardiac remodeling in obesity. Physiological Reviews, 88, 389–419.
Barouch, L. A., Gao, D., Chen, L., et al. (2006). Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circulation Research, 98, 119–124.
Silvani, A., Bastianini, S., Berteotti, C., Franzini, C., Lenzi, P., Lo Martire, V., et al. (2009). Sleep modulates hypertension in leptin-deficient obese mice. Hypertension, 53, 251–255.
Buchanan, J., Mazumder, P. K., Hu, P., et al. (2005). Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology, 146, 5341–5349.
Christoffersen, C., Bollano, E., Lindegaard, M. L. S., Bartels, E. D., Goetze, J. P., Andersen, C. B., et al. (2003). Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology, 144, 3483–3490.
Brindley, D. N., Kok, B. P. C., Kienesberger, P. C., Lehner, R., & Dyck, J. R. B. (2010). Shedding light on the enigma of myocardial lipotoxicity: the involvement of known and putative regulators of fatty acid storage and mobilization. American Journal of Physiology, Endocrinology and Metabolism, 298, E897–E908.
Park, T. S., Yamashita, H., Blaner, W. S., & Goldberg, I. J. (2007). Lipids in the heart: a source of fuel and a source of toxins. Current Opinion in Lipidology, 18, 277–282.
Bugger, H., & Abel, E. D. (2008). Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clinical Science, 114, 195–210.
Chiu, H. C., Kovacs, A., Ford, D. A., et al. (2001). A novel mouse model of lipotoxic cardiomyopathy. Journal of Clinical Investigation, 107, 813–822.
Boudina, S., Sena, S., O'Neill, B. T., Tathireddy, P., Young, M. E., & Abel, E. D. (2005). Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation, 112, 2686–2695.
Boudina, S., Sena, S., Theobald, H., et al. (2007). Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes, 56, 2457–2466.
Aronis, A., Madar, Z., & Tirosh, O. (2005). Mechanism underlying oxidative stress-mediated lipotoxicity: exposure of J774.2 macrophages to triacylglycerols facilitates mitochondrial reactive oxygen species production and cellular necrosis. Free Radical Biology & Medicine, 38, 1221–1230.
Bielawska, A. E., Shapiro, J. P., Jiang, L., et al. (1997). Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. American Journal of Pathology, 151, 1257–1263.
Kong, J. Y., Klassen, S. S., & Rabkin, S. W. (2005). Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Molecular and Cellular Biochemistry, 278, 39–51.
Parra, V., Eisner, V., Chiong, M., et al. (2008). Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovascular Research, 77, 387–397.
Listenberger, L. L., Han, X. L., Lewis, S. E., Cases, S., Farese, R. V., Ory, D. S., et al. (2003). Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 100, 3077–3082.
de Vries, J. E., Vork, M. M., Roemen, T., de Jong, Y. F., Cleutjens, J., Van Der Vusse, G., et al. (1997). Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. Journal of Lipid Research, 38, 1384.
Weiss, E. P., & Fontana, L. (2011). Caloric restriction: powerful protection for the aging heart and vasculature. American Journal of Physiology-Heart and Circulatory Physiology, 301, H1205–H1219.
Shinmura, K., Tamaki, K., Sano, M., Murata, M., Yamakawa, H., Ishida, H., et al. (2011). Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. Journal of Molecular and Cellular Cardiology, 50, 117–127.
Viljanen, A. P. M., Karmi, A., Borra, R., et al. (2009). Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. The American Journal of Cardiology, 103, 1721–1726.
Hammer, S., Snel, M., Lamb, H. J., et al. (2008). Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. Journal of the American College of Cardiology, 52, 1006–1012.
Sloan, C., Tuinei, J., Nemetz, K., et al. (2011). Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes, 60, 1424–1434.
Rame, J. E., Barouch, L. A., Sack, M. N., et al. (2011). Caloric restriction in leptin deficiency does not correct myocardial steatosis: failure to normalize PPAR alpha/PGC1 alpha and thermogenic glycerolipid/fatty acid cycling. Physiological Genomics, 43, 726–738.
Breslow, M. J., Min-Lee, K., Brown, D. R., Chacko, V. P., Palmer, D., & Berkowitz, D. E. (1999). Effect of leptin deficiency on metabolic rate in ob/ob mice. American Journal of Physiology, Endocrinology and Metabolism, 276, E443–E449.
Morricone, L., Malavazos, A. E., Coman, C., Donati, C., Hassan, T., & Caviezel, F. (2002). Echocardiographic abnormalities in normotensive obese patients: relationship with visceral fat. Obesity Research, 10, 489–498.
Münzel, T., Afanas’ev, I. B., Kleschyov, A. L., & Harrison, D. G. (2002). Detection of superoxide in vascular tissue. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 1761–1768.
Moens, A. L., Leyton-Mange, J. S., Niu, X., et al. (2009). Adverse ventricular remodeling and exacerbated NOS uncoupling from pressure-overload in mice lacking the beta3-adrenoreceptor. Journal of Molecular and Cellular Cardiology, 47, 576–585.
Takimoto, E., Champion, H. C., Li, M., et al. (2005). Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. The Journal of Clinical Investigation, 115, 1221–1231.
Bielawski, J., Szulc, Z. M., Hannun, Y. A., & Bielawska, A. (2006). Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods, 39, 82–91.
Birse, R. T., & Bodmer, R. (2011). Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Critical Reviews in Biochemistry and Molecular Biology, 46, 376–385.
Korosoglou, G., Humpert, P. M., Ahrens, J., et al. (2012). Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. Journal of Magnetic Resonance Imaging, 35, 804–811.
Sikka, G., Yang, R., Reid, S., et al. (2010). Leptin is essential in maintaining normal vascular compliance independent of body weight. International Journal of Obesity, 34, 203–206.
Kaltman, A. J., & Goldring, R. M. (1976). Role of circulatory congestion in the cardiorespiratory failure of obesity. American Journal of Medicine, 60, 645–653.
Lakatta, E. G. (1987). Do hypertension and aging have a similar effect on the myocardium? Circulation, 75, I69–I77.
Ren, J., Dong, F., Cai, G. J., Zhao, P., Nunn, J. M., Wold, L. E., et al. (2010). Interaction between age and obesity on cardiomyocyte contractile function: role of leptin and stress signaling. PLoS One, 5, e10085.
Zhen, J., Lu, H., Wang, X. Q., Vaziri, N. D., & Zhou, X. J. (2008). Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. American Journal of Hypertension, 21, 28–34.
Luo, J., Xuan, Y. T., Gu, Y., & Prabhu, S. D. (2006). Prolonged oxidative stress inverts the cardiac force-frequency relation: role of altered calcium handling and myofilament calcium responsiveness. Journal of Molecular and Cellular Cardiology, 40, 64–75.
Balaban, R. S., Nemoto, S., & Finkel, T. (2005). Mitochondria, oxidants, and aging. Cell, 120, 483–495.
Dai, D. F., Rabinovitch, P. S., & Ungvari, Z. (2012). Mitochondria and cardiovascular aging. Circulation Research, 110, 1109–1124.
Bakris, G. L., Bank, A. J., Kass, D. A., Neutel, J. M., Preston, R. A., & Oparil, S. (2004). Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. American Journal of Hypertension, 17, 23s–30s.
Liu, L., Shi, X., Bharadwaj, K. G., et al. (2009). DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. Journal of Biological Chemistry, 284, 36312–36323.
Hernández-Corbacho, M. J., Jenkins, R. W., Clarke, C. J., Hannun, Y. A., Obeid, L. M., Snider, A. J., et al. (2011). Accumulation of long-chain glycosphingolipids during aging is prevented by caloric restriction. PLoS One, 6, e20411.
Wu, M. Z., Katta, A., Gadde, M. K., et al. (2009). Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. Plos One, 4(7), e6430.
Funding
The authors are grateful for the financial support of the American Heart Association Beginning Grant-In-Aid [to L.A.B.], American Diabetes Association [to L.A.B.], and the National Institutes of Health [5T32HL007227 to V.L.W]. There are no relationships to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AlGhatrif, M., Watts, V.L., Niu, X. et al. Beneficial Cardiac Effects of Caloric Restriction Are Lost with Age in a Murine Model of Obesity. J. of Cardiovasc. Trans. Res. 6, 436–445 (2013). https://doi.org/10.1007/s12265-013-9453-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9453-4