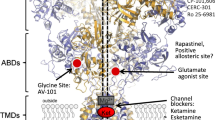
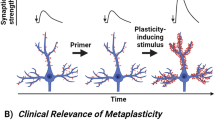
Abstract
Depression is a devastating psychiatric disorder widely attributed to deficient monoaminergic signaling in the central nervous system. However, most clinical antidepressants enhance monoaminergic neurotransmission with little delay but require 4–8 weeks to reach therapeutic efficacy, a paradox suggesting that the monoaminergic hypothesis of depression is an oversimplification. In contrast to the antidepressants targeting the monoaminergic system, a single dose of the N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine produces rapid (within 2 h) and sustained (over 7 days) antidepressant efficacy in treatment-resistant patients. Glutamatergic transmission mediated by NMDARs is critical for experience-dependent synaptic plasticity and learning, processes that can be modified indirectly by the monoaminergic system. To better understand the mechanisms of action of the new antidepressants like ketamine, we review and compare the monoaminergic and glutamatergic antidepressants, with emphasis on neural plasticity. The pathogenesis of depression may involve maladaptive neural plasticity in glutamatergic circuits that may serve as a new class of targets to produce rapid antidepressant effects.
Similar content being viewed by others
References
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013, 34: 119–138.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289: 3095–3105.
Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med 2001, 7: 541–547.
Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008, 358: 55–68.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry 2006, 163: 1905–1917.
Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 2006, 7: 137–151.
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 2012, 71: 939–946.
Berman R M, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Anti-depressant effects of ketamine in depressed patients. Biol Psychiatry 2000, 47: 351–354.
Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 2000, 61Suppl 6: 4–6.
Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology 2011, 36: 2589–2602.
Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci 2002, 22: 3638–3644.
LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012, 491: 594–598.
Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry 2012, 72: 537–547.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-Daspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006, 63: 856–864.
Huttenlocher PR. Neural plasticity. Harvard University Press, 2009.
Lenn NJ. Neuro plasticity: The basis for brain development, learning, and recovery from injury. Infants Young Child 1991, 3: 39–48.
McGaugh JL. Memory—a century of consolidation. Science 2000, 287: 248–251.
Zhang M. Autism Disease: Neural network going awry and therapeutic Sstrategy underlying neural plasticity. N A J Med Sci 2011, 4: 139.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 2008, 33: 88–109.
Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 2007, 8: 844–858.
Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 2003, 54: 338–352.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008, 455: 894–902.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004, 56: 640–650.
MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 2003, 100: 1387–1392.
Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 2002, 51: 708–714.
Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci 2003, 985: 420–444.
Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biol Psychiatry 2006, 60: 966–973.
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 2007, 61: 198–209.
Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 2007, 33: 1909–1918.
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007, 27: 8877–8884.
Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res 2002, 136: 443–453.
Hashimoto K, Sawa A, Iyo M. I ncreased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 2007, 62: 1310–1316.
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 1999, 46: 1375–1387.
Neumaier JF, Root DC, Hamblin M W. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 1996, 15: 515–522.
Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry 1996, 153: 174–182.
Billings AG, Cronkite RC, Moos RH. Social-environmental factors in unipolar depression: comparisons of depressed patients and nondepressed controls. J Abnorm Psychol 1983, 92: 119–133.
Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci 2012, 13: 478–490.
Magariños AM, Verdugo JM, McEwen B S. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A 1997, 94: 14002–14008.
Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997, 17: 2492–2498.
McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012, 62: 3–12.
Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A 2009, 106: 14075–14079.
Pittenger C, Sanacora G, Krystal JH. Th e NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets 2007, 6: 101–115.
Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62: 63–77.
Mallei A, Giambelli R, Gass P, Racagni G, Mathé AA, Vollmayr B, et al. Synaptoproteomics of learned helpless rats involve energy metabolism and cellular remodeling pathways in depressive-like behavior and antidepressant response. Neuropharmacology 2011, 60: 1243–1253.
Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One 2010, 5: e8566.
Popoli M, Yan Z, McEwen BS, Sanacora G. Th e stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012, 13: 22–37.
Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry 2013, 73: 1180–1188.
Hajszan T, MacLusky NJ, Leranth C. Short-ter m treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci 2005, 21: 1299–1303.
Norrholm SD, Ouimet CC. Altered dendritic spin e density in animal models of depression and in response to antidepressant treatment. Synapse 2001, 42: 151–163.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011, 69: 754–761.
Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 2006, 59: 1136–1143.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chron ic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000, 20: 9104–9110.
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476: 458–461.
Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol 1987, 48: 138–149.
Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 1997, 387: 497–500.
Seligman ME. Learned helplessness. Annu Rev Med 1972, 23: 407–412.
Beck A, Rush A, Shaw B, Emery G. Cognitive therapy of depression, 1979. Guilford, New York.
Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry 2007, 164: 516–519.
Gorwood P, Corruble E, Falissard B, Goodwin GM. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry 2008, 165: 731–739.
Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull 2010, 26: 327–337.
Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci 1997, 9: 637–642.
Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. St ress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 1996, 58: 1475–1483.
Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron 2002, 36: 527–538.
de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998, 394: 787–790.
Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast 2007, 2007.
Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces as sociative long-term potentiation in the amygdala. Nature 1997, 390: 604–607.
Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science 2006, 313: 1093–1097.
Vyas A, Mitra R, Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002, 22: 6810–6818.
Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Ágústsdóttir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 2011, 334: 1731–1734.
Naughton M, Mulrooney JB, Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol 2000, 15: 397–415.
Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 1996, 29: 2–11.
Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology 2008, 33: 2080–2092.
McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci 1999, 22: 295–318.
Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 2005, 136: 29–37.
Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 2007, 61: 187–197.
Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 2010, 15: 80–92.
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002, 22: 3251–3261.
Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A 2006, 103: 13208–13213.
Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006, 59: 1116–1127.
Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, et al. Inductio n of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 1994, 79: 49–58.
Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 1994, 79: 59–68.
Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, et al. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell 1995, 83: 979–992.
Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 2010, 33: 230–240.
Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 1997, 88: 615–626.
Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science 2001, 294: 1030–1038.
Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1998, 1: 602–609.
Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated prote in kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci 2000, 20: 6317–6325.
Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto GM, et al. Requirement of ERK activation for visual cortical plasticity. Science 2001, 292: 2337–2340.
Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, et al. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 2001, 32: 123–140.
Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 2002, 22: 3673–3682.
Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiat 2003, 60: 273–282.
Yamada S, Yamamoto M, Ozawa H, Riederer P, Saito T. Reduced phosphorylation of cyclic AM P-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J Neural Transm 2003, 110: 671–680.
Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry 2001, 49: 753–762.
Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, et al. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase I V and MAP kinase cascades. Neuropsychopharmacology 2004, 29: 1831–1840.
Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, et al. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci 2000, 20: 4030–4036.
Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther 1993, 265: 1380–1386.
Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci 2005, 25: 3270–3279.
Bobula B, Tokarski K, Hess G. Repeated administration of antidepressants decreases field potentials in rat frontal cortex. Neuroscience 2003, 120: 765–769.
Tokarski K, Bobula B, Wabno J, Hess G. Repeated administration of imipramine attenuates glutamatergic transmission in rat frontal cortex. Neuroscience 2008, 153: 789–795.
Albo F, Pieri M, Zona C. Modulation of AMPA receptors in spinal motor neurons by the neuroprotective agent riluzole. J Neurosci Res 2004, 78: 200–207.
Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 2007, 32: 793–802.
Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac). Proc Natl Acad Sci U S A 2002, 99: 3182–3187.
Cai X, Kallarackal AJ, Kvarta MD, Goluskin S, Gaylor K, Bailey AM, et al. Local potentiation of ex citatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci 2013, 16: 464–472.
Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem 1984, 42: 1–11.
Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 2011, 16: 156–170.
Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces bi phasic PSANCAM expression in the adult rat dentate gyrus. Eur J Neurosci 2003, 17: 879–886.
Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A 1997, 94: 14002–14008.
Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 2001, 7: S71–80.
Tokita K, Yamaji T, Hashimoto K. Roles of glutamate signaling in preclinical and/or mechanistic models o f depression. Pharmacol Biochem Behav 2012, 100: 688–704.
Monteggia LM, Malenka RC, Deisseroth K. Depression: the best way forward. Nature 2014, 515: 200–201.
Ibrahim L, DiazGranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improveme nt in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 2012, 37: 1526–1533.
Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav 2002, 71: 341–344.
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 2013, 74: 250–256.
Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 2010, 167: 1305–1320.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329: 959–964.
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 2008, 63: 349–352.
Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 2008, 32: 140–144.
Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 2009, 30: 563–569.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977, 229: 327–336.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013, 38: 729–742.
Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 2003, 112: 631–643.
Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, et al. Emotion enhances learning via norepinephrine regulat ion of AMPA-receptor trafficking. Cell 2007, 131: 160–173.
Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentia tor (LY392098). Neuropharmacology 2001, 40: 1028–1033.
Mackowiak M, O’Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology 2002, 43: 1–10.
Alt A, Witkin JM, Bleakman D. AMPA receptor potentiators as novel antidepressants. Curr Pharm Des 2005, 11: 1511–1527.
Chen JJ, Xu L, Zhou J, Lv J, Mao RR, Tian M, et al. The application of 5-methyl-1,3-benzenebiol or derivatives thereof in the preparation of medicines and functional foods for treatment or prevention of depression. Asia, Australia and Africa Patent No. 200710066088.7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Jing, L., Toledo-Salas, JC. et al. Rapid-onset antidepressant efficacy of glutamatergic system modulators: The neural plasticity hypothesis of depression. Neurosci. Bull. 31, 75–86 (2015). https://doi.org/10.1007/s12264-014-1484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-014-1484-6