Abstract
Vitamin D receptor polymorphisms may predispose that not all individuals could have benefits from the nutritional supplementation of 25-hydroxyvitamin D. Furthermore, vitamin D-related cardiovascular effects may also be influenced by soy isoflavones considered endocrine regulators of cardiovascular homeostasis. To find possible gene–diet interactions by evaluating individualized lipid metabolism benefits from an increase in soy and 25-hydroxyvitamin D intake, 106 healthy individuals, genotyped for vitamin D receptor (VDR) gene polymorphism rs1544410 (BsmI) were randomly assigned to either no intake, to daily 250 mL or 500 mL of a 25-hydroxyvitamin D supplemented SB for 2 months. The soybean beverage induced differences in cardiovascular risk factors (lipid profile, blood pressure, TNFα and MCP-1), as well as vitamin D metabolites in a dose-gene-dependent relation. Thus, VDR BsmI polymorphism affected individual response being the GG genotype the ones that showed dose-dependent manner responsiveness in the reduction in total cholesterol, LDL and triglycerides in comparison with the AA/AG genotype. These differences were associated with increased plasma levels of 1α,25-dyhydroxyvitamin D3 in the carriers of the GG genotype. It was concluded that metabolic response to 25-hydroxyvitamin D and soybean supplementation is dependent on VDR BsmI GG genotype due to a higher conversion rate from vitamin D precursors.
Similar content being viewed by others
Introduction
Soy intake has been regarded as a one of the principal nutritional interventions for the prevention and treatment of cardiovascular diseases, principally because of its capacity to control glucose (Azadbakht et al. 2007), lipid homeostasis (Hermansen et al. 2005) and the regulation of inflammation (Miraghajani and Azadbakht 2011). However, some discrepancies in the results have been reported in the literature with respect to the net effects of soy intake in metabolism biomarkers. Recently, a meta-analysis has shown a lack of significant overall effect of soy intake on improvements of fasting glucose and insulin concentrations (Liu et al. 2011), whereas other meta-analyses on serum lipoproteins have shown that regular consumption of soy protein daily (15–30 g) has a significant favorable impact on serum lipoprotein and risk factors for coronary heart disease (Anderson and Bush 2011), while other studies have not found any effect (Matthan et al. 2007). The reasons for these discrepancies can be the heterogeneity of soybean preparations—this food per se is a complex mixture of several nutrients and other known and unknown compounds—as well as the degree of responsiveness between subjects. This individual response capacity is due to differences in the metabolization of its compounds (Hall et al. 2006) and possibly to diverse interactions of isoflavones and other bioactive compounds with several receptors controlling transcriptional responses to nutrient and non-nutrient substances in soy.
Between the factors that control genetic transcription with relationship to metabolic syndrome and cardiovascular diseases, recently emphasis has been placed on the role of vitamin D in areas beyond bone metabolism and calcium homeostasis. As vitamin D receptor (VDR) gene is expressed in a large number of tissues, it is not surprising that ligand-activated VDR modulates expression of multiple targeted genes (Makariou et al. 2011), which is argued with the fact that vitamin D deficiency has been associated with risk factors for cardiovascular disease, metabolic syndrome and even with overall mortality (Schöttker et al. 2012).
The endocrine actions of the active form of vitamin D (1α,25(OH)2D3) are mediated both by genetic and nongenetic pathways. The former are activated by the binding of 1α,25(OH)2D3 to a specific cytosolic/nuclear VDR, a member of the steroid/thyroid hormone receptor superfamily (Wang et al. 2012). The human VDR gene is located on chromosome 12q12–q14, and five common polymorphisms have been typically associated with VDR activity (Audí et al. 2004; Capoluongo et al. 2006; Chang et al. 2000; García et al. 2007; Panierakis et al. 2009; Taverna et al. 2002), namely Cdx2 (rs11568820), FokI (rs10735810), TaqI (rs731236), BsmI (rs1544410) and ApaI (rs7975232). The Cdx2 polymorphism is located in the promoter region of the VDR gene, with the A allele giving higher transcriptional activity of the gene. The FokI RFLP is located in the coding region of the VDR gene and leads to the production of a VDR protein that is three amino acids longer. Although no significant differences in ligand affinity, DNA binding or transactivation activity have been found between the two allelic FokI forms, the shorter RFLP variant display higher activity of the receptor than the longer one. Last, the BsmI, ApaI and TaqI RFLPs are located in the 3’ end of the gene. BsmI does not alter the structure or function of the final VDR protein produced, but it is strongly linked with a poly (A) repeat and may affect VDR messenger RNA stability (Vuolo et al. 2012).
A large number of studies have been published which have investigated the association between these four polymorphisms with several diseases but the results have been inconclusive. BsmI polymorphism has been associated with hyperparathyroidism (Carling et al. 1995) and osteocalcin levels (Morrison et al. 1994), which was further related to bone mineral density. Related to cardiovascular disease, the B allele (G allele) of the BsmI genotype has been associated with higher levels of blood pressure in healthy men (Muray et al. 2003) but not associated with the prevalence and severity of coronary artery disease (Ortlepp et al. 2003). Therefore, it has been suggested that the overall effect of the polymorphism may be strongly influenced by some other nongenetic factors like diet.
In this line, vitamin D status is well known to be affected by dietary factors. Moreover, several studies and clinical practices have shown that estrogen and vitamin D can be effective in the management of postmenopausal metabolic changes. Likewise, soy isoflavones, which shares structural similarity with estrogen, have reported to induce hormonal changes in postmenopausal women (Hooper et al. 2009). However, relatively few studies have investigated the interaction of soy intake with vitamin D metabolic effects. Therefore, in this study, it was evaluated the effect of the daily supplementation with a soy beverage (SB) enriched with 25-hydroxyvitamin D (25(OH)D3) for 2 months in cardiovascular disease risk factors, accounting the BsmI VDR polymorphism (rs1544410) as a possible source for patient responsiveness.
Materials and methods
Soybean beverage description
The SB employed in the study is commercially available in Spain (Grupo Leche Pascual, Aranda de Duero, Spain). The nutritional composition is shown in Table S1 (Supplemental material), depicting the nutritional label described in the product. This SB is fortified in vitamin A & D (retinol and 25(OH)-hydroxycholecalciferol, respectively). The product was presented in individual bricks of 250 mL of content.
Subjects and study design
A total of 106 nonsmoker individuals (68 women and 38 men with an average age of 33.7 ± 12.2) were recruited through brochure distribution within the University community and research group website. At the baseline, participants were randomly assigned to the Soybean beverage 1 (SB1: 250 mL/day), Soybean beverage 2 (SB2: 500 mL/day) or the control group. Baseline characteristics of volunteers are described in Table S2 (Supplemental material). No statistical differences in anthropometrics and biochemical parameters were found between groups. Subjects were allowed to consume their regular diet along the study, and daily dietary recalls of soybean beverage intake was measured to determine treatment adherence. Blood samples for biochemical determination, blood pressure and anthropometrics information were collected in fasted subjects at the baseline (11 h, 16 min daylight duration) and at week 8 (14 h, 01 min daylight duration) of experimentation. Subjects gave their informed consent to participate in the intervention, and all experimental procedures were in agreement with the ethical standards in accordance with the policy statements of the institutional review board of University of Lleida (Catalonia-Spain).
Blood biochemical and anthropometric analysis
Blood samples were collected in 12-h overnight fasting subjects by venipunction into Vacutainer® tubes. Total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides were determined in the Biochemical Analysis Services of the Hospital Universitari Arnau de Vilanova (Lleida, Spain). TNFα & MCP-1 were determined using a xMAP Milliplex kits (Human Metabolic Hormone Panel Cat. #HMH-34K Millipore) following manufacturer instructions. Height and weight was recorder at baseline and after 8 weeks of the beginning of the intervention period. Blood pressure was measured using an electronic sphygmomanometer (OMROM, M6 Comfort, OMROM Health Co, Japan).
Serum vitamin D metabolites determination
Serum vitamin D metabolites were determined by UHPLC-MS/MS following the procedure described by Aronov et al. (2008). Interday and intraday CV was below 2.5 and 5 %, respectively.
VDR BsmI (rs1544410) genotype determination
DNA was isolated from buccal epithelial cells, obtained using BuccalAmp swabs (Epicenter BioTechnologies, Madison, Wisconsin). DNA samples were later processed according to manufacturer’s instructions. Validated TaqMan assay for SNPs rs1544410 (also known as BsmI) was purchased from Applied Biosystems (Carlsbad, USA) and used for the determination of individual genotypes, as described elsewhere (McGuigan and Ralston 2002). The iCycler’s Allelic Discrimination Software analyzed the fluorescence levels in each sample and automatically classified the sample in one of two groups, depending on the presence/absence of allele A (also known in the literature as allele B): Group A consisted of Homozygous AA and Heterozygous AG, and represented around 60 % of the total sample. Group G contained the Homozygous GG, around 40 % of individuals of the analyzed sample. These frequencies are consistent with HapMap CEU allele frequencies for this polymorphism (65 vs. 35 %) and are also consistent with the expected allele and genotype frequencies for a European population under Hardy–Weinberg equilibrium.
Statistics
Statistical analysis was conducted using SPSS version 17.0 for Windows. Normality of variables was checked using Kolmogorov–Smirnov test. One way ANOVA or paired t Student test was performed within each one of the groups for the different parameters determined, with post hoc analyses when appropriate. Differences with p < 0.05 were considered significant. Data are presented as mean ± SD for the number of subjects in each group.
Results
Soy beverage intake effects on cardiovascular disease risk factors and vitamin D metabolites profile
The SB treatment was prescribed to be taken ad libitum once or twice a day at the study period in the SB1 and SB2 groups, respectively. Most volunteers in the SB1 group reported that the main period of intake was during the morning, while volunteers from the SB2 group reported one dose in the morning and the other in the afternoon. The mean adherence to the treatment of the volunteers, measured by dietary recall, was as high as 90 % in both SB1 and SB2 groups.
Although the high degree of adherence reported, the effect of 2-month consumption of the SB, without any nutritional intervention or advice, was minor in relation to measured cardiovascular disease risk factors (Table 1). Slight changes in the lipid profile and selected inflammation biomarkers were observed.
In relation to vitamin D plasma metabolites, as expected, a significant increase in the levels of 25(OH)D3 was observed in SB1 and SB2 groups. The observed change in the pre-activated form of the vitamin D was not confounded by seasonal changes, since the control group showed a reduction in its plasma levels (not statistically significant) through daylight duration increased. Moreover, the increase in 25(OH)D3 contents may corroborate the adherence of the soy treatment in both experimental groups since the soy beverage was supplemented with 25(OH)D3 (Table S1).
Few reports in the literature have found any evidence of the increase in the plasma levels of the pre-activated form of vitamin D after soy supplementation. Some authors suggest that isoflavones, like genistein, may increase and decrease the activity of the CYP27B1 and CYP24 enzymes, respectively, and therefore modify the plasma levels of 1α,25(OH)2D3 and 24R,25(OH)2D3 (Cross et al. 2004). However, no reports have been found about the effects of soy or soy compounds in the activity of CYP27A and CYP27B, responsible for the first hydroxylation of cholecalciferol in the liver. Taking all together, it seems that the increase in the levels of 25(OH)D3 could be mainly explained by its higher oral intake provided by the soy beverage.
Influence of VDR BsmI (rs1544410) alleles in cardiovascular risk factors profile: basal and response to SB intake
BsmI polymorphism is located near the 3’UTR region of the VDR gene, which has been reported to be involved in the regulation of the expression of VDR, especially through regulation of mRNA stability (Decker and Parker 1995; Morrison et al. 1994). According to the HapMap-CEU allele distribution for Europeans, similar results have been found in this study with respect to A and G allele distribution, being the A allele the one with the higher incidence of around 60 % of the study population.
At the baseline of the study, no differences were found between the carriers of the two different alleles in the plasma lipid profile, vitamin D metabolites, the selected inflammation biomarkers and blood pressure (Fig. 1), except to a slightly higher level in triglyceride found in the carriers of the G allele. These suggest that the BsmI polymorphism has not a major influence in parameters of cardiovascular disease risk. Notwithstanding, it should be noted that the number of subjects included in the study is relatively low. In the same way, no reports relating this polymorphism with cardiovascular disease risk parameter have been found in the literature, implying that BsmI may not predispose to a higher cardiovascular disease risk.
Differences in cardiovascular risk factors between VDR rs1544410 polymorphism alleles at the beginning of the study, a Lipid profile, b plasma vitamin D metabolites, c selected inflammatory biomarkers and d blood pressure. Only differences in the levels of triglycerides between the two alleles were observed at the beginning of the study, being the volunteers from the G allele and the ones with a higher level. One way ANOVA was performed for the different parameters analyzed
However, after 2 months of SB intake, the carriers of the G allele showed a higher grade of responsiveness to the treatment, especially in blood lipid profile. In these carriers, it was found a significant reduction in the levels of total cholesterol (Fig. 2a), LDL cholesterol (Fig. 2b) and HDL cholesterol (Fig. 2c) (p < 0.013, p < 0.032 and p < 0.049 for VDR according 2 way ANOVA controlling VDR and treatment) and a not significant reduction in the triglycerides levels (Fig. 2d). Moreover, in relation to blood pressure, it was observed differences in the relationships exhibited between systolic and diastolic blood pressures and plasma concentrations of 24R,25(OH)2D3 (Fig. 3) between the two allelic types. Thus, while highly significant inverse correlations were present in the A allele group, these relationships were lost in the G allele group.
Net effects of soy beverage in blood lipid profile after 2 months of treatments in volunteers grouped by BsmI (rs1544410) polymorphism. a Total cholesterol, b LDL cholesterol, c LDL cholesterol and d triglycerides. Statistically differences were found in the reduction in the levels of total cholesterol and LDL-cholesterol in the SB2 group with the G allele. Both control and SB1 groups with the A allele showed an increase in the levels of HDL cholesterol. Paired t Student test was performed within each one of the groups for the different parameters analyzed
Correlation between the blood pressure and the levels of 24R,25(OH)2D3 between subjects with different VDR BsmI (rs1544410) alleles. A significant correlation was observed between the levels of 24R,25(OH)2D3 and systolic and diastolic blood pressure in subjects with the A allele (a and b) (p = 0.0082 and 0.0002, respectively), whereas the subjects with the G allele (c and d) showed no correlation (p = 0.1864 and 0.6971, respectively)
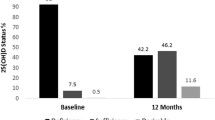
In addition to the observed relationship, a change in plasma vitamin D metabolites (Fig. 4) was also observed between the two types of alleles, where an increase in the 25(OH)D3 (p < 0.05 for treatment by 2-way ANOVA) and activated form of vitamin D (Fig. 3c) (p < 0.01 for treatment by 2-way ANOVA) without a major reduction in the 24R,25(OH)2D3 (Fig. 3b) was reported. The behavior observed in the parameters described showed a dose response effect between the different doses of soy beverage. Accordingly, an opposite effect was observed in the carriers of the A allele, which infer that the net effects of the SB intake could be explained in part due to differences in VDR BsmI polymorphism. In this context, it seems that the volunteers from the G allele may obtain more benefit from the supplementation of SB than the carriers of the A allele, in a relationship with a higher amount of activated vitamin D.
Net changes in vitamin D metabolite concentrations after 2 months of soy beverage supplementation between subjects with different VDR BsmI (rs1544410) alleles. No changes were observed in the pre-activated form of vitamin D3 (a), whereas a reduction in 24R,25(OH)2D3 (b) and an increase in the activated form (c) were observed in subjects with the G allele. Statistically differences were found in the increase in the activated form of vitamin D3 in subjects in the SB2 group with the G allele compared to control subjects with the G allele. Almost no differences were observed due the treatment in subjects with the A allele
One of the possible explanations of the differences in the effect between alleles could be explained in part due to the differences in the levels of the activated form of vitamin D in the G allele group (Fig. 3c). For instance, it has been reported that the intraperitoneal injection of the activated form of vitamin D (1α,25(OH)2D3) modulates the expression of enzymes involved in lipid homeostasis; mainly down-regulating FAS and ACC, and up-regulating CPT-1 and PPARα (Yin et al. 2012), explaining the lower doses of cholesterol and triglycerides observed in the carriers of the G allele. Notwithstanding, although vitamin D deficiency is associated with an unfavorable lipid profile in cross-sectional analyses, the supplementation of 25(OH)D3 or its increase in plasma concentrations is not associated with a improvement in lipid profile as it has been described in several studies (Ponda et al. 2012; Wood et al. 2012). Same findings are observed in this study, where the supplementation of the pre-activated form of vitamin D did not show any improvement in the lipid profile in the whole population studied (Table 1). In the same way, the carriers of the A allele which showed an increase in the levels of 25(OH)D3 (Fig. 3a) did not show an improvement in the lipid profile.
Complementarily, as shown in Fig. 3d, there is a significant correlation between the levels of the activated and 24R,25(OH)2D3 forms of vitamin D in A allele group, which is considered to be homeostatically controlled since both metabolites are inversely regulated by the VDR, CYP27B1 and CYP24 enzymes and other related hormones (Anderson et al. 2004). This correlation is lost in the carriers of the G allele, where an increase in the levels of both metabolites is observed, suggesting that VDR cannot regulate the changes in the concentrations of plasma vitamin D metabolites. In this context, it is proposed that the beneficial effects of SB supplementation in the carriers of the G allele could be mainly attributed to the resulting differences in the levels of the activated form of vitamin D.
Discussion
Soybean, a most food items, is a complex mixture of compounds that may include known nutrients and bioactive compounds and unknown compounds not properly characterized. This feature makes it difficult to attribute a food’s observed effect to a single compound. In this sense, despite the effect of soy and soy compounds in health has been widely studied, in the clinical practice, different response to its treatment has been observed between subjects. These differences have in some cases been attributed to different isoflavone metabolization processes during colonic fermentation (Jacobs et al. 2009), as well as gender and age (Ko et al. 2010) and some genetic variants, for example, involved in lipid metabolism (Torres et al. 2009). However, even that gene–disease association has been well characterized in some cases; there still is few evidence of the effect of nutritional intervention in individuals from the different variants described. In addition, most of the genetic variants taking into account are based on phenotypic differences found before interventional studies and not based in the individually determined differences in response due to genetic variance.
In this context, results from this study, without taking into consideration the genetic variability of the volunteers, showed a great variance in the changes in lipid profile between subjects; after statistic analysis of the data, it was concluded that 2 months of soy beverage treatment did not confer a major effect in the lipid profile as shown in Table 1.
Notwithstanding, one of the main findings of this study is the description of the possible interaction between soy consumption and vitamin D metabolism. The results from this study show that some of the effects of soy treatment can be explained because of its effects in vitamin D metabolites, which may depend on the sensitivity to vitamin D signaling determined by the polymorphisms in its receptor.
Since the expression of CYP27B1 and CYP24 toward the production of the activated and 24R,25(OH)2D3 forms of vitamin D, respectively, may depend on the circulating levels of the different forms of vitamin D, it is necessary to point out that the expression of these enzymes is induced after the activation of the VDR (Schwartz et al. 2001). In this context, as it is hypothesized in Fig. 5, high levels of 1α,25(OH)2D3, recognized by VDR, induce a higher expression of the CYP24 enzyme in direction to reduce its levels producing a higher amount of 24R,25(OH)2D3. In the same way, lower levels of the activated form of vitamin D may increase the expression of the CYP27B1 enzyme toward an increase in 1α,25(OH)2D3. This homeostatic behavior seems to be lost in the carriers of the G allele and may explain the increase in the levels of the activated form of vitamin D after the chronic consumption of SB.
Homeostatic regulation of vitamin D metabolites controlled by VDR and the influence of VDR polymorphism BsmI transcriptional activity. Soy compounds together with higher plasma amounts of 25(OH)D3 may induce a higher conversion of the activated form of vitamin D (1). The increase in 1α,25(OH)2D3, recognized by the VDR (2), reduction in the expression of CYP27B1 and increase in the expression of CYP24 (3) modifying the plasma levels of 1α,25(OH)2D3 and 24R,25(OH)2D3 (4). This homeostatic regulation is not effective in volunteers of the G allele BsmI polymorphism, which is recognized to induce a low-stability RNA and therefore changes in the VDR activity. In this context, the increase in 1α,25(OH)2D3 induced by soy compounds and vitamin D (5) is not effectively regulated by VDR (6), suggesting that the expression of the CYP27B1 and CYP24 enzymes are not controlled (7), inducing higher plasma levels of 1α,25(OH)2D3 (8)
In this manner, it is possible to argue that the volunteers of the G allele, which transcript a low stability VDR mRNA and hence a low content of VDR (Morrison et al. 1994), may have a low response to plasma modifications of vitamin D levels. The cellular differences in the content of VDR could induce to a higher and lower levels of the activated and 24R,25(OH)2D3 forms of vitamin D as it is observed in Fig. 3c, b, respectively, circumstance not observed in volunteers from the A allele. Therefore, volunteers with the G allele could be benefited either by increased production or decreased destruction of vitamin D active form induced by soybean compounds. The later question to be responded is how the soybean intake induces a higher plasma content of the activated form of vitamin D and the implications and influence of extra-renal tissues involved in the activation of vitamin D, e.g., small intestine enterocytes with direct contact with soy beverage compounds.
References
Anderson JW, Bush HM (2011) Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr 30:79–91
Anderson PH, O’Loughlin PD, May BK, Morris HA (2004) Determinants of circulating 1,25-dihydroxyvitamin D3 levels: the role of renal synthesis and catabolism of vitamin D. J Steroid Biochem Mol Biol 89–90:111–113
Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD (2008) Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 391:1917–1930
Audí L, Martí G, Esteban C, Oyarzabal M, Chueca M, Gussinyé M, Yeste D, Fernández-Cancio M, Andaluz P, Carrascosa A (2004) VDR gene polymorphism at exon 2 start codon (Fokl) may have influenced Type 1 diabetes mellitus susceptibility in two Spanish populations [1]. Diabet Med 21:393–394
Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC (2007) Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr 85:735–741
Capoluongo E, Pitocco D, Concolino P, Santonocito C, Di Stasio E, d’Onofrio G, Manto A, Giardina B, Ghirlanda G, Ameglio F, Zuppi C (2006) Slight association between type 1 diabetes and “ff” VDR FokI genotype in patients from the Italian Lazio Region. Lack of association with diabetes complications. Clin Biochem 39:888–892
Carling T, Kindmark A, Hellman P, Lundgren E, Ljunghall S, Rastad J, Akerstrom G, Melhus H (1995) Vitamin D receptor genotypes in primary hyperparathyroidism. Nat Med 1:1309–1311
Chang T-, Lei H-, Yeh J-, Chiu KC, Lee K-, Chen M-, Tai T-, Chuang L- (2000) Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clin Endocrinol (Oxf) 52:575–580
Cross HS, Kállay E, Lechner D, Gerdenitsch W, Adlercreutz H, Armbrecht HJ (2004) Phytoestrogens and vitamin D metabolism: a new concept for the prevention and therapy of colorectal, prostate, and mammary carcinomas. J Nutr 134:1207S–1212S
Decker CJ, Parker R (1995) Diversity of cytoplasmic functions for the 3′ untranslated region of eukaryotic transcripts. Curr Opin Cell Biol 7:386–392
García D, Angel B, Carrasco E, Albala C, Santos JL, Pérez-Bravo F (2007) VDR polymorphisms influence the immune response in type 1 diabetic children from Santiago, Chile. Diabetes Res Clin Pract 77:134–140
Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft H-F, Ferrari M, Branca F, Dadd T, Talbot D, Powell J, Minihane A-, Cassidy A, Nilsson M, Dahlman-Wright K, Gustafsson J-, Williams CM (2006) Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr 83:592–600
Hermansen K, Hansen B, Jacobsen R, Clausen P, Dalgaard M, Dinesen B, Holst JJ, Pedersen E, Astrup A (2005) Effects of soy supplementation on blood lipids and arterial function in hypercholesterolemic subjects. Eur J Clin Nutr 59:843–850
Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A (2009) Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update 15:423–440
Jacobs DM, Gaudier E, van Duynhoven J, Vaughan EE (2009) Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Curr Drug Metab 10:41–54
Ko T-F, Tsai H-S, Lin S-M, Liu C-D, Learn S-P, Chiou RY (2010) GC-MS determined distribution of urinary equol producers as affected by age, gender, and repeated ingestions of soymilk. J Food Sci 75:H306–H310
Liu ZM, Chen Y-, Ho SC (2011) Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr 93:1092–1101
Makariou S, Liberopoulos EN, Elisaf M, Challa A (2011) Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med 22:355–362
Matthan NR, Jalbert SM, Ausman LM, Kuvin JT, Karas RH, Lichtenstein AH (2007) Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr 85:960–966
McGuigan FEA, Ralston SH (2002) Single nucleotide polymorphism detection: allelic discrimination using TaqMan. Psychiatr Genet 12:133–136
Miraghajani MS, Azadbakht L (2011) Can soy products affect on inflammation level? A review on the current evidence. J Isfahan Med Sch 29:1116–1128
Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook PN, Eisman JA (1994) Prediction of bone density from vitamin D receptor alleles. Nature 367:284–287
Muray S, Parisi E, Cardús A, Craver L, Fernández E (2003) Influence of vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D on blood pressure in apparently healthy subjects. J Hypertens 21:2069–2075
Ortlepp JR, Von Korff A, Hanrath P, Zerres K, Hoffmann R (2003) Vitamin D receptor gene polymorphism BsmI is not associated with the prevalence and severity of CAD in a large-scale angiographic cohort of 3441 patients. Eur J Clin Invest 33:106–109
Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E (2009) Vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Crete, Greece. Clin Immunol 133:276–281
Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW (2012) Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation 126:270–277
Schöttker B, Ball D, Gellert C, Brenner H (2012) Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev 12:708–718
Schwartz Z, Pedrozo HA, Sylvia VL, Gomez R, Dean DD, Boyan BD (2001) 1α,25-(OH)2D3 regulates 25-hydroxyvitamin D3 24R-hydroxylase activity in growth zone costochondral growth plate chondrocytes via protein kinase C. Calcif Tissue Int 69:365–372
Taverna MJ, Sola A, Guyot-Argenton C, Pacher N, Bruzzo F, Slama G, Reach G, Selam J- (2002) Taq I polymorphism of the vitamin D receptor and risk of severe diabetic retinopathy. Diabetologia 45:436–442
Torres N, Guevara-Cruz M, Granados J, Vargas-Alarcón G, González-Palacios B, Ramos-Barragan VE, Quiroz-Olguín G, Flores-Islas IM, Tovar AR (2009) Reduction of serum lipids by soy protein and soluble fiber is not associated with the ABCG5/G8, apolipoprotein E, and apolipoprotein A1 polymorphisms in a group of hyperlipidemic Mexican subjects. Nutr Res (New York, N.Y.) 29:728–735
Vuolo L, Di Somma C, Faggiano A, Colao A (2012) Vitamin D and cancer. Front Endocrinol (Lausanne) 3:58
Wang Y, Zhu J, DeLuca HF (2012) Where is the vitamin D receptor? Arch Biochem Biophys 523:123–133
Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM (2012) Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab 97(10):3557–3568
Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W (2012) Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest 42(11):1189–1196
Acknowledgments
This study was supported by grants from the Spanish Ministry of Science and Innovation (CENIT MET-DEV-FUN) to M. Portero-Otín, the Spanish Instituto de Salud Carlos III (FIS PI081843) to M. Portero-Otin.
Conflict of interest
Jose C. E. Serrano, David De Lorenzo, Anna Cassanye, Meritxell Martín-Gari, Reinald Pamplona and Manuel Portero-Otin declare that they have no conflict of interest. Alberto Espinel and Marco Antonio Delgado are employees of the soybean beverage manufacturer company Grupo Leche Pascual.
Ethical standards
All experimental procedures were in agreement with the ethical standards in accordance with the policy statements of the institutional review board of University of Lleida (Catalonia-Spain) and with the Helsinki Declaration of 1975. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Serrano, J.C.E., De Lorenzo, D., Cassanye, A. et al. Vitamin D receptor BsmI polymorphism modulates soy intake and 25-hydroxyvitamin D supplementation benefits in cardiovascular disease risk factors profile. Genes Nutr 8, 561–569 (2013). https://doi.org/10.1007/s12263-013-0356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12263-013-0356-4