Abstract
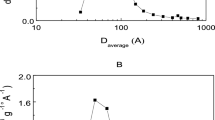
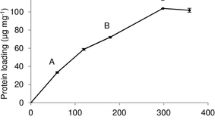
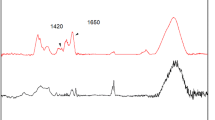
Efficient biological degradation of lignocellulosic biomass is a key step towards developing sustainable biorefineries. One potential approach to improve process efficiency is the use of enzyme immobilization to facilitate reuse of the enzymes. Two different commercial cellulase containing enzyme preparations, termed Cellulases 1 (C1) and Cellulases 2 (C2) for the purposes of this manuscript, were separately immobilized on non-porous silica (S1) and porous silica (S2). The effect of pH, ionic strength and enzyme loading on enzyme stability and activity were all investigated. Immobilized cellulases on S1 showed equivalent enzyme activity as free cellulases, and those on S2 retained 60% enzyme activity. Dissociation of the cellulases from the support after immobilization and during hydrolysis was found to be minimal, suggesting strong enzyme-support interactions. Further, Scanning Electron Microscopy images revealed that S1 and S2 containing immobilized cellulases differentially interact with cellulose, which likely contributes to the observed differences in hydrolysis.
Similar content being viewed by others
References
Koutinas, A. A., C. Du, and R. H. Wang (2008) Production of chemicals from biomass. pp. 77–101. In: J. Clark and F. Deswarte (eds.). Introduction to Chemicals from Biomass. John Wiley, A John Wiley & Sons, Ltd., Cornwall, UK.
Wall, J. D., C. S. Harwood, and A. Demain (2008) Bioenergy. ASM Press, VA, USA.
Turley, D. B. (2008) The chemical value of biomass. pp. 21–46. In: J. Clark and F. Deswarte (eds.). Introduction to Chemicals from Biomass. John Wiley, A John Wiley & Sons, Ltd., Cornwall, UK.
Cao, L. (2005) Adsorption-based Immobilization, Carrier-bound Immobilized Enzymes pp. 53–54. 1st ed. WILEY-VCH Verlag GmbH & Co. KGaA, Morlenbach, Germany.
Dinçer, A. and A. Telefoncu (2007) Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B- Enz. 45: 10–14.
Mousdale, D. M. (2008) Biofuels: Biotechnology, Chemistry, and Sustainable Development. CRC Press, Taylor & Francis Group, LLC., FL, USA.
Yang, S., W. Ding, and H. Chen (2009) Enzymatic hydrolysis of corn stalk in a hollow fiber ultrafiltration membrane reactor. Biomass Bioenerg. 33: 332–336.
Hartono, S. B., S. Z. Qiao, J. Liu, K. Jack, B. P. Ladewig, Z. Hao, and G. Q. M. Lu (2010) Functionalized mesoporous silica with very large pores for cellulase immobilization. J. Phys. Chem. C. 114: 8353–8362.
Iler, R. K. (1979) Polymerization of Silica, The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry. pp. 174–175. A Wiley-Interscience Publication/John Wiley & Sons, Cornwall, UK.
Cruz, J. C., P. H. Pfromm, and M. E. Rezac (2009) Immobilization of Candida antarctica lipase B on fumed silica. Proc. Biochem. 44: 62–69.
Ikeda, Y. (2013) Cellulase immobilizations with highly retained enzymatic activities. Ph. D. Thesis. University of Alberta, Edmonton, Alberta, Canada.
Ghose, T. K. (1987) Measurement of cellulase activities. Pure Appl. Chem. 59: 257–268.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72: 248–254.
Miller, G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428.
Zhu, L., J. P. O’Dwyer, V. S. Chang, C. B. Granda, and M. T. Holtzapple (2008) Structural features affecting biomass enzymatic digestibility. Bioresour. Technol. 99: 3817–3828.
Tébéka, I. R. M., A. G. L. Silva, and D. F. S. Petti (2009) Hydrolytic activity of free and immobilized cellulase. Langmuir 25: 1582–1587.
Henriksson, H., J. Ståhlberg, R. Isaksson, and G. Pettersson (1996) The active sites of cellulases are involved in chiral recognition: A comparison of cellobiohydrolase 1 and endoglucanase 1. FEBS Lett. 390: 339–344.
Takimoto, A., T. Shiomi, K. Ino, T. Tsunoda, A. Kawai, F. Mizukami, and K. Sakaguchi (2008) Encapsulation of cellulase with mesoporous silica (SBA-15). Micropor. Mesopor. Mat. 116: 601–606.
Chang, R. H. Y., J. Jang, and K. C. W. Wu (2011) Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose- to-glucose conversion. Green Chem. 13: 2844–2850.
Tamaru, Y., H. Miyake, K. Kuroda, M. Ueda, and R. H. Doi (2010) Comparative genomics of the mesophilic cellulosome-producing clostridium cellulovorans and its application to biofuel production via consolidated bioprocessing. Environ. Technol. 31: 889–903.
Park, E. Y., P. N. Anh, and N. Okuda (2004) Bioconversion of waste office paper to L(+)-lactic acid by the filamentous fungus Rhizopus oryzae. Bioresour. Technol. 93: 77–83.
Liang, W. and X. Cao (2012) Preparation of a pH-sensitive polyacrylate amphiphilic copolymer and its application in cellulase immobilization. Bioresour. Technol. 116: 140–146.
Yu, A., J. Yuan, Q. Wang, X. Fan, P. Wang, and X. Sun (2012) Covalent immobilization of cellulases onto a water-soluble-insoluble reversible polymer. Biochem. Biotechnol. 166: 1433–1441.
Alahakoon, T., J. W. Koh, X. W. C. Chong, and W. T. L. Lim (2012) Immobilization of cellulases on amine and aldehyde functionalized Fe2O3 magnetic nanoparticles. Prep. Biochem. Biotechnol. 42: 234–248.
Zhang, Y., J. L. Xu, Z. H. Yuan, W. Qi, Y. Liu, and M. C. He (2012) Artificial intelligence techniques to optimize the EDC/NHS-mediated immobilization of cellulase on Eudragit L-100. Int. J. Mol. Scis. 13: 7952–7962.
Xu, Z., Y. Miao, J. Y. Chen, X. Jiang, L. Lin, and P. Ouyang (2011) Co-immobilization mechanism of cellulase and xylanase on a reversibly soluble polymer. Appl. Biochem. Biotechnol. 163: 153–161.
Xu, J., S. Huo, Z. Yuan, Y. Zhang, H. Xu, Y. Guo, C. Liang, and X. Zhuang (2011) Characterization of direct cellulase immobilization with super paramagnetic nanoparticles. Biocatal. Biotransfor. 29: 71–76.
Zhou, J. (2010) Immobilization of cellulase on a reversibly soluble- insoluble support: Properties and application. J. Agr. Food Chem. 58: 6741–6746.
Liao, H., D. Chen, L. Yuan, M. Zheng, Y. Zhu, and X. Liu (2010) Immobilized cellulase by polyvinyl alcohol/Fe2O3 magnetic nanoparticle to degrade microcrystalline cellulose. Carbohyd. Polym. 82: 600–604.
Afsahi, B., A. Kazemi, A. Kheirolomoom, and S. Nejati (2007) Immobilization of cellulase on non-porous ultrafine silica particles. Sci. Iran. 14: 379–383.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikeda, Y., Parashar, A. & Bressler, D.C. Highly retained enzymatic activities of two different cellulases immobilized on non-porous and porous silica particles. Biotechnol Bioproc E 19, 621–628 (2014). https://doi.org/10.1007/s12257-014-0191-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0191-5