Abstract
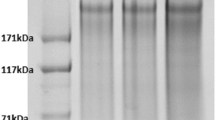
Cocoon sericin plays an important role in the reeling of silk and serves as a valuable biomaterial in the field of biomedicine, skincare, and food industries; however, knowledge about cocoon sericin proteins has been limited. For a comprehensive study on sericin, cocoons of eight varieties of silkworm of different geographic origin and with varied cocoon color were analyzed utilizing proteomics and bioinformatics approaches. The electrophoresis pattern demonstrated some common protein bands for all silkworm varieties and distinctive protein bands for some of those examined in the present study. The Ser2 protein, a new Ser3 protein, and four other novel sericin proteins were identified in cocoons for the first time. Products of both Ser1 and Ser3 genes appear to be ubiquitous in the cocoon shell of Bombyx mori. In addition, cocoons with especially high-reelability produced by the mutant strain B84 had an unique protein product of the Ser2 gene, indicating that the protein may play an important role in cocoon reelability. A series of sequence conflicts and post-translational modifications (PTMs) were also revealed in sericin proteins. Lipid modifications of sericin proteins, which promote waterproofing of the cocoon shell, were observed. Further, hydroxylation was identified, which provided evidence for intermolecular bonds among neighboring molecules of sericin as found in collagens. The sericin proteome data obtained from this study illuminated the molecular complexity of cocoon sericin and contributed to our understanding of the properties of sericin in filature and biomaterials.
Similar content being viewed by others
References
Kundu, S. C., B. C. Dash, R. Dash, and D. L. Kaplan (2008) Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 33: 998–1012.
Zhang, Y. Q. (2002) Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 20: 91–100.
Gamo, T., T. Inokuchi, and H. Laufer (1977) Polypeptides of fibroin and sericin secreted from different sections of silk gland in Bombyx Mori. Insect Biochem. 7: 285–295.
Sprague, K. U. (1975) The Bombyx mori silk proteins: Characterization of large polypeptides. Biochem. 14: 925–931.
Takasu, Y., H. Yamada, and K. Tsubouchi (2002) Isolation of three main sericin components from the cocoon of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 66: 2715–2718.
Takasu, Y., H. Yamada, T. Tamura, H. Sezutsu, K. Mita, and K. Tsubouchi (2007) Identification and characterization of a novel sericin gene expressed in the anterior middle silk gland of the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 37: 1234–1240.
Takasu, Y., T. Hata, K. Uchino, and Q. Zhang (2010) Identification of Ser2 proteins as major sericin components in the noncocoon silk of Bombyx mori. Insect Biochem. Mol. Biol. 40: 339–344.
Tokutake, S. (1980) Isolation of the smallest component of silk protein. Biochem. J. 187: 413–417.
Garel, A., G. Deleage, and J. C. Prudhomme (1997) Structure and organization of the Bombyx mori sericin 1 gene and of the sericins 1 deduced from the sequence of the Ser 1B cDNA. Insect Bioche. Mol. Biol. 27: 469–477.
Michaille, J. J., P. Couble, J. C. Prudhomme, and A. Garel (1986) A single gene produces multiple sericin messenger-RNAs in the silk gland of Bombyx mori. Biochimie. 68: 1165–1173.
Okamoto, H., E. Ishikawa, and Y. Suzuki (1982) Structural analysis of sericin genes. Homologies with fibroin gene in the 5’ flanking nucleotide sequences. J. Biol. Chem. 257: 15192–15199.
Takasu, Y., H. Yamada, and K. Tsubouchi (2006) The silk sericin component with low crystallinity. Sanshi-Konchu Biotec. 75: 133–139.
Teramoto, H., A. Kakazu, and T. Asakura (2006) Native structure and degradation pattern of silk sericin studied by C-13 NMR spectroscopy. Macromol. 39: 6–8.
Zhang, P., Y. Aso, K. Yamamoto, Y. Banno, Y. Wang, K. Tsuchida, Y. Kawaguchi, and H. Fujii (2006) Proteome analysis of silk gland proteins from the silkworm, Bombyx mori. Proteom. 6: 2586–2599.
Aramwit, P., S. Damrongsakkul, S. Kanokpanont, and T. Srichana (2010) Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol. Appl. Biochem. 55: 91–98.
Martel, A., M. Burghammer, R. J. Davies, and C. Riekel (2007) Thermal behavior of Bombyx mori silk: Evolution of crystalline parameters, molecular structure, and mechanical properties. Biomacromol. 8: 3548–3556.
Teramoto, H., K. Nakajima, and C. Takabayashi (2004) Chemical modification of silk sericin in lithium chloride/dimethyl sulfoxide solvent with 4-cyanophenyl lsocyanate. Biomacromol. 5: 1392–1398.
Teramoto, H. and M. Miyazawa (2005) Molecular orientation behavior of silk sericin film as revealed by ATR infrared spectroscopy. Biomacromol. 6: 2049–2057.
Teramoto, H., A. Kakazu, K. Yamauchi, and T. Asakura (2007) Role of hydroxyl side chains in Bombyx mori silk sericin in stabilizing its solid structure. Macromol. 40: 1562–1569.
Zhang, Y. Q., M. L. Tao, W. D. Shen, Y. Z. Zhou, Y. Ding, Y. Ma, and W. L. Zhou (2004) Immobilization of L-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomat. 25: 3751–3759.
Takasu, Y., H. Yamada, and K. Tsubouchi (2002) Extraction and chromatographic analysis of cocoon sericin of the silkworm, Bombyx mori. J. Insect Biotechnol. Sericol. 71: 151–155.
Laemmli, U. K. (1970) Cleavage of structureal proteins during assenmbly of head of bacteriophage-T4. Nature 227: 680–685.
Fernandez, J., F. Gharahdaghi, and S. M. Mische (1998) Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using matrix assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS). Electrophoresis 19: 1036–1045.
Gamo, T. (1973) Electrophoretic analyses of the protein extracted with disulfide cleavage from cocoons of the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 42: 17–23.
Johnston, D. A., S. Capetillo, L. S. Ramagli, J. Guevara, D. M. Gersten, and L. V. Rodriguez (1984) Standardization of protein position in Silver-stained two-dimensional polyacrylamide-gel electrophoresis. Electrophoresis 5: 110–116.
Tabunoki, H., S. Higurashi, O. Ninagi, H. Fujii, Y. Banno, M. Nozaki, M. Kitajima, N. Miura, S. Atsumi, K. Tsuchida, H. Maekawa, and R. Sato (2004) A carotenoid-binding protein (CBP) plays a crucial role in cocoon pigmentation of silkworm (Bombyx mori) larvae. FEBS Lett. 567: 175–178.
Tamura, Y., K. Nakajima, K. Nagayasu, and C. Takabayashi (2002) Flavonoid 5-glucosides from the cocoon shell of the silkworm, Bombyx mori. Phytochem. 59: 275–278.
Kurioka, A. and M. Yamazaki (2002) Purification and identification of flavonoids from the yellow green cocoon shell (Sasamayu) of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 66: 1396–1399.
Nakajima, M. (1963) Physiological studies on the function of genes concerning carotenoid permeability in the silkworm. Bull. Fac. Agric. Tokyo Univ. Agric. Technol. 8: 1–80.
Aramwit, P., S. Kanokpanont, T. Nakpheng, and T. Srichana (2010) The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 11: 2200–2211.
Altman, G. H., F. Diaz, C. Jakuba, T. Calabro, R. L. Horan, J. Chen, H. Lu, J. Richmond, and D. L. Kaplan (2003) Silk-based biomaterials. Biomat. 24: 401–416.
Panilaitis, B., G. H. Altman, J. Chen, H. J. Jin, V. Karageorgiou, and D. L. Kaplan (2003) Macrophage responses to silk. Biomaterials 24: 3079–3085.
Wen, C. M., S. T. Ye, L. X. Zhou, and Y. Yu (1990) Silk-induced asthma in children — a report of 64 cases. Ann. Allergy 65: 375–378.
Chen, W. Q., H. Priewalder, J. P. P. John, and G. Lubec (2010) Silk cocoon of Bombyx mori: Proteins and posttranslational modifications — heavy phosphorylation and evidence for lysine-mediated cross links. Proteom. 10: 369–379.
Miwa, N., K. Yokoyama, H. Wakabayashi, and N. Nio (2010) Effect of deamidation by protein-glutaminase on physicochemical and functional properties of skim milk. Int. Dairy J. 20: 393–399.
Winkler, S., D. Wilson, and D. L. Kaplan (2000) Controlling beta-sheet assembly in genetically engineered silk by enzymatic phosphorylation/dephosphorylation. Biochem. 39: 12739–12746.
Stewart, R. J. and C. S. Wang (2010) Adaptation of caddisily larval silks to aquatic habitats by phosphorylation of H-fibroin serines. Biomacromol. 11: 969–974.
Magee, A. I., L. Gutierrez, I. A. McKay, C. J. Marshall, and A. Hall (1987) Dynamic fatty acylation of P21N-RAS. Embo. J. 6: 3353–3357.
Minoura, N., S. Aiba, Y. Gotoh, M. Tsukada, and Y. Imai (1995) Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 29: 1215–1221.
Huang, J., R. Valluzzi, E. Bini, B. Vernaglia, and D. L. Kaplan (2003) Cloning, expression, and assembly of sericin-like protein. J. Biol. Chem. 278: 46117–46123.
Wu, W., W. J. Li, L. Q. Wang, K. H. Tu, and W. L. Sun (2006) Synthesis and characterization pH- and temperature-sensitive sericin/poly(N-isopropylacrylamide) interpenetrating polymer networks. Polym. Int. 55: 513–519.
Tao, W., M. Z. Li, and R. J. Xie (2005) Preparation and structure of porous silk sericin materials. Macromol. Mater. Eng. 290: 188–194.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, X., Li, J. & Chen, Y. Proteomic analysis of sericin in Bombyx mori cocoons. Biotechnol Bioproc E 16, 438–444 (2011). https://doi.org/10.1007/s12257-010-0425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-010-0425-0