Abstract
Background
Protein phosphatase 1 α (PP1A) is an enzyme intimately associated with cell cycle, the over expression of which has been demonstrated in glioblastoma (GBM). Further, the nuclear expression of PP1A has been shown to be highly specific to GBM. In addition, PP1A has been shown to be a connecting molecule in the p53 containing GBM sub network. In view of these, we evaluated the prognostic relevance of PP1A.
Methods
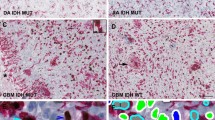
GBM tissues were examined for protein expression of PP1A by immunohistochemistry (IHC). Nuclear expression of PP1A was scored in all tumor tissue samples. Survival analyses were performed by Cox-Regression and Kaplan-Meier survival analysis with Log Rank tests. IDH1, ATRX and p53 IHC and stratification of all GBM cases were performed and subgroup specific evaluation of nuclear PP1A correlation with overall and progression free survival was performed.
Results
PP1A protein expression showed no correlation with prognosis in all cases of GBM or on stratification based on IDH1 or ATRX expression. However on p53 stratification nuclear PP1A expression emerged as strong independent predictor of poor overall survival only in p53 positive GBMs both in univariate and multivariate analysis.
Conclusions
While PP1A expression uniquely associates with poor prognosis only in p53 expressing GBMs, there is a notable absence of such correlation in p53 negative GBMs; thus skewing the overall relation of this molecule with prognosis in GBM. PP1A emerging as a strong prognostic marker in p53 expressing GBMs, enables us to foresee this molecule as a potential therapeutic target.


Similar content being viewed by others
References
Omuro A, DeAngelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA 310:1842–50. doi:10.1001/jama.2013.280319
Ladha J, Donakonda S, Agrawal S et al (2010) Glioblastoma-specific protein interaction network identifies PP1A and CSK21 as connecting molecules between cell cycle-associated genes. Cancer Res 70:6437–47. doi:10.1158/0008-5472.CAN-10-0819
Ceulemans H, Bollen M (2004) Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84:1–39. doi:10.1152/physrev.00013.2003
Wu JQ, Guo JY, Tang W et al (2009) PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol 11:644–51. doi:10.1038/ncb1871
Wang RH, Liu CW, Avramis VI, Berndt N (2001) Protein phosphatase 1alpha-mediated stimulation of apoptosis is associated with dephosphorylation of the retinoblastoma protein. Oncogene 20:6111–22. doi:10.1038/sj.onc.1204829
Castro ME, Ferrer I, Cascón A et al (2008) PPP1CA contributes to the senescence program induced by oncogenic Ras. Carcinogenesis 29:491–9. doi:10.1093/carcin/bgm246
Lu Z, Wan G, Guo H et al (2013) Protein phosphatase 1 inhibits p53 signaling by dephosphorylating and stabilizing Mdmx. Cell Signal 25:796–804. doi:10.1016/j.cellsig.2012.12.014
Ruiz L, Traskine M, Ferrer I et al (2008) Characterization of the p53 response to oncogene-induced senescence. PLoS One 3:e3230. doi:10.1371/journal.pone.0003230
Thota B, Shukla SK, Srividya MR et al (2012) IDH1 mutations in diffusely infiltrating astrocytomas: grade specificity, association with protein expression, and clinical relevance. Am J Clin Pathol 138:177–84. doi:10.1309/AJCPZOIY3WY4KIKE
Haberler C, Wöhrer A (2014) Clinical Neuropathology practice news 2–2014: ATRX, a new candidate biomarker in gliomas. Clin Neuropathol 33:108–111. doi:10.5414/NP300758
Hou LC, Veeravagu A, Hsu AR, Tse VCK (2006) Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus 20:E5
Lamborn KR, Yung WKA, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–70. doi:10.1215/15228517-2007-062
Weller M, Felsberg J, Hartmann C et al (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27:5743–50. doi:10.1200/JCO.2009.23.0805
Srividya MR, Thota B, Arivazhagan v et al (2010) Age-dependent prognostic effects of EGFR/p53 alterations in glioblastoma: study on a prospective cohort of 140 uniformly treated adult patients. J Clin Pathol 63:687–91. doi:10.1136/jcp.2009.074898
Hofstetter CP, Burkhardt J-K, Shin BJ et al (2012) Protein phosphatase 2A mediates dormancy of glioblastoma multiforme-derived tumor stem-like cells during hypoxia. PLoS One 7:e30059. doi:10.1371/journal.pone.0030059
Lu J, Kovach JS, Johnson F et al (2009) Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc Natl Acad Sci U S A 106:11697–702. doi:10.1073/pnas.0905930106
Li J, Huang J, Li M et al (2012) Anisomycin induces glioma cell death via down-regulation of PP2A catalytic subunit in vitro. Acta Pharmacol Sin 33:935–40. doi:10.1038/aps.2012.46
Tsaytler P, Bertolotti A (2013) Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. FEBS J 280:766–70. doi:10.1111/j.1742-4658.2012.08535.x
Cohen PTW (2002) Protein phosphatase 1–targeted in many directions. J Cell Sci 115:241–56
Chatterjee J, Köhn M (2013) Targeting the untargetable: recent advances in the selective chemical modulation of protein phosphatase-1 activity. Curr Opin Chem Biol 17:361–8. doi:10.1016/j.cbpa.2013.04.008
Reddy SP, Britto R, Vinnakota K et al (2008) Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res 14:2978–87. doi:10.1158/1078-0432.CCR-07-4821
Pich A, Margaria E, Chiusa L (2000) Oncogenes and male breast carcinoma: c-erbB-2 and p53 coexpression predicts a poor survival. J Clin Oncol 18:2948–56
Nakopoulou LL, Alexiadou A, Theodoropoulos GE et al (1996) Prognostic significance of the co-expression of p53 and c-erbB-2 proteins in breast cancer. J Pathol 179:31–8. doi:10.1002/(SICI)1096-9896(199605)179:1<31::AID-PATH523>3.0.CO;2-O
Park YB, Kim HS, Oh JH, Lee SH (2001) The co-expression of p53 protein and P-glycoprotein is correlated to a poor prognosis in osteosarcoma. Int Orthop 24:307–10
Xie Y, Bulbul MA, Ji L et al (2014) p53 expression is a strong marker of inferior survival in de novo diffuse large B-cell lymphoma and may have enhanced negative effect with MYC coexpression: a single institutional clinicopathologic study. Am J Clin Pathol 141:593–604. doi:10.1309/AJCPPHMZ6VHF0WQV
Takami H, Yoshida A, Fukushima S et al (2014) Revisiting TP53 mutations and immunohistochemistry—a comparative study in 157 diffuse gliomas. Brain Pathol. doi:10.1111/bpa.12173
Liu CWY, Wang R-H, Berndt N (2006) Protein phosphatase 1alpha activity prevents oncogenic transformation. Mol Carcinog 45:648–56. doi:10.1002/mc.20191
Hsu L-C, Huang X, Seasholtz S et al (2006) Gene amplification and overexpression of protein phosphatase 1alpha in oral squamous cell carcinoma cell lines. Oncogene 25:5517–26. doi:10.1038/sj.onc.1209563
Acknowledgments
We acknowledge Prof. M.R.S Rao, Jawaharlal Nehru Centre for Advanced Scientific Research for his support and guidance. Project investigators and project assistants, involved NIMITLI-CSIR (Neuro-Oncology) project are acknowledged. Arun H Shastry is supported by Indian Council of Medical Research, Government of India, under the MD-PhD/TSS-2, medical scientist training program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shastry, A.H., Thota, B., Srividya, M.R. et al. Nuclear Protein Phosphatase 1 α (PP1A) Expression is Associated with Poor Prognosis in p53 Expressing Glioblastomas. Pathol. Oncol. Res. 22, 287–292 (2016). https://doi.org/10.1007/s12253-015-9928-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-015-9928-5