Abstract
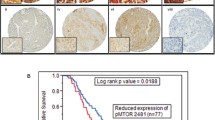
As mammalian Target of Rapamycin (mTOR) plays role in protein synthesis and metabolism, mTOR pathway activation is involved in the pathogenesis of several types of tumors. Our aim was to elucidate its role in medulloblastoma in terms of prognosis and as a therapeutic target. Members of activated mTOR complex 1 (mTORC1) pathway, phospho-mTOR (p-mTOR) and phospho-S6 (p-S6) were examined by immunohistochemistry in formalin fixed paraffin embedded samples of 40 patients with medulloblastoma, and results were compared to clinical features and survival of patients. In proliferation assays, Daoy and UW228–2 medulloblastoma cell lines were tested by rapamycin, an mTORC1 inhibitor, and NVP-BEZ235, a dual mTOR and phosphatidylinositol 3-kinase (PI3K) inhibitor, each in monotherapy and in combination with cytostatic drugs (cisplatin, etoposide). Components of mTORC1 and mTORC2 complexes were also examined in these cell lines. Neither presence of p-mTOR (32.5 %) nor p-S6 (32.5 %) correlated with age, gender or histological subtype. In 22.5 % of cases simultaneous expression of p-mTOR and p-S6 was shown. Kaplan-Meier analysis showed inferior survival of patients expressing both marker proteins, but it was not statistically significant, probably due to low case number. UW228–2 cells had greater sensitivity to mTOR inhibitors, possibly due to its higher mTORC1 specific protein expression levels, compared to Daoy cells. In both cell lines antiproliferative effect of cytostatic drugs was significantly enhanced by mTOR inhibitors (p < 0.05). Based on our in vitro and clinicopathological studies mTOR inhibitors may have a role in the future treatment of a subset of patients with medulloblastoma.




Similar content being viewed by others
References
Hatten ME, Roussel MF (2011) Development and Cancer of the Cerebellum. Trends Neurosci 34:134–142
Roussel MF, Hatten ME (2011) Cerebellum Development and Medulloblastoma. Curr Top Dev Biol 94:235–282
Crawford JR, MacDonald TJ, Packer RJ (2007) Medulloblastoma in Childhood: new Biological Advances. Lancet Neurol 6:1073–1085
Massimino M, Giangaspero F, Garrè ML, Gandola L, Poggi G, Biassoni V, Gatta G, Rutkowski S (2011) Childhood Medulloblastoma. Crit Rev Oncol Hematol 79:65–83
Gottardo NG, Gajjar A (2008) Chemotherapy for Malignant Brain Tumors of Childhood. J Child Neurol 23:1149–1159
Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A (2013) Survival and Secondary Tumors in Children with Medulloblastoma Receiving Radiotherapy and Adjuvant Chemotherapy: Results of Children’s Oncology Group trial A9961. Neuro Oncol 15:97–103
Dazert E, Hall MN (2011) mTOR Signaling in Disease. Curr Opin Cell Biol 23:744–755
Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D (2012) PI3K and mTOR Signaling Pathways in Cancer: New Data on Targeted Therapies. Curr Oncol Rep 14:129–138
Guertin DA, Sabatini DM (2007) Defining the Role of mTOR in Cancer. Cancer Cell 12:9–22
Dunlop EA, Tee AR (2009) Mammalian target of Rapamycin Complex 1: Signalling Inputs, Substrates and Feedback Mechanisms. Cell Signal 21:827–835
Akcakanat A, Singh G, Hung MC, Meric-Bernstam F (2007) Rapamycin Regulates the Phosphorylation of Rictor. Biochem Biophys Res Commun 362:330–333
Fouladi M, Laningham F, Wu J, O’Shaughnessy MA, Molina K, Broniscer A, Spunt SL, Luckett I, Stewart CF, Houghton PJ, Gilbertson RJ, Furman WL (2007) Phase I Study of Everolimus in Pediatric Patients with Refractory Solid Tumors. J Clin Oncol 25:4806–4812
Jozwiak J, Sontowska I, Bikowska B, Grajkowska W, Galus R, Roszkowski M (2011) Favourable Prognosis in Medulloblastoma with Extensive Nodularity is Associated with Mitogen-activated Protein Kinase Upregulation. Folia Neuropathol 49:257–261
Wlodarski P, Grajkowska W, Lojek M, Rainko K, Jozwiak J (2006) Activation of Akt and Erk Pathways in Medulloblastoma. Folia Neuropathol 44:214–220
Wlodarski PK, Boszczyk A, Grajkowska W, Roszkowski M, Jozwiak J (2008) Implication of Active Erk in the Classic Type of Human Medulloblastoma. Folia Neuropathol 46:117–122
Bhatia B, Northcott PA, Hambardzumyan D, Govindarajan B, Brat DJ, Arbiser JL, Holland EC, Taylor MD, Kenney AM (2009) Tuberous Sclerosis Complex Suppression in Cerebellar Development and Medulloblastoma: Separate Regulation of Mammalian Target of Rapamycin Activity and p27 Kip1 Localization. Cancer Res 69:7224–7234
Bhatia B, Nahlé Z, Kenney AM (2010) Double Trouble: when Sonic Hedgehog Signaling Meets TSC Inactivation. Cell Cycle 9:456–459
Mainwaring LA, Kenney AM (2011) Divergent Functions for eIF4E and S6 Kinase by Sonic Hedgehog Mitogenic Signaling in the Developing Cerebellum. Oncogene 30:1784–1797
Parathath SR, Mainwaring LA, Fernandez-L A, Campbell DO, Kenney AM (2008) Insulin Receptor Substrate 1 is an Effector of Sonic Hedgehog Mitogenic Signaling in Cerebellar Neural Precursors. Development 135:3291–3300
Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, Hsiao K, Yuan J, Green J, Ospina B, Yu Q, Ostrom L, Fordjour P, Anderson DL, Monahan JE, Kelleher JF, Peukert S, Pan S, Wu X, Maira SM, Garcia-Echeverria C, Briggs KJ, Watkins DN, Yao YM, Lengauer C, Warmuth M, Sellers WR, Dorsch M (2010) Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med 2:51ra70
Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, Phillips PC, Janss AJ (2001) Antitumor Activity of the Rapamycin Analog CCI-779 in Human Primitive Neuroectodermal Tumor/Medulloblastoma Models as Single Agent and in Combination Chemotherapy. Cancer Res 61:1527–1532
Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, Witt H, Korshunov A, Read TA, Sun JL, Schmitt EM, Miller CR, Buckley AF, McLendon RE, Westbrook TF, Northcott PA, Taylor MD, Pfister SM, Febbo PG, Wechsler-Reya RJ (2012) An animal Model of MYC-driven Medulloblastoma. Cancer Cell 21:155–167
Hauser P, Jakab Z, Kiss C, Szegedi I, Bárdi E, Batyik K, Ottóffy G, Kajtár P, Szűcs R, Nagy K, Cservák J, Masát P, Bálint K, Kordás M, Bognár L, Kocsis B, Vízkeleti J, Kriván G, Kállay K, Benyó G, Schuler D, Garami M (2009) Előzetes eredmények a medulloblastoma/primitív neuroektodermális tumor (PNET) kezelésében a magyar MBL 2004 kezelési sémával. Magyar Belorvosi Archívum, pp 196–201
Back JH, Kim AL (2011) The expanding Relevance of Nuclear mTOR in Carcinogenesis. Cell Cycle 10:3849–3852
Malik AR, Urbanska M, Macias M, Skalecka A, Jaworski J (2013) Beyond Control of Protein Translation: what we Have Learned About the Non-canonical Regulation and Function of Mammalian Target of Rapamycin (mTOR). Biochim Biophys Acta 1834:1434–1448
Rosner M, Hengstschläger M (2008) Cytoplasmic and Nuclear Distribution of the Protein complexes mTORC1 and mTORC2: Rapamycin Triggers Dephosphorylation and Delocalization of the mTORC2 Components riCtor and Sin1. Hum Mol Genet 17:2934–2948
Jozwiak J, Grajkowska W, Wlodarski P (2007) Pathogenesis of Medulloblastoma and Current Treatment Outlook. Med Res Rev 27:869–890
Yecies JL, Manning BD (2011) mTOR links Oncogenic Signaling to Tumor Cell Metabolism. J Mol Med (Berl) 89:221–228
Del Valle L, Enam S, Lassak A, Wang JY, Croul S, Khalili K, Reiss K (2002) Insulin-like Growth Factor I Receptor Activity in Human Medulloblastomas. Clin Cancer Res 8:1822–1830
Yang J, Liao D, Wang Z, Liu F, Wu G (2011) Mammalian Target of Rapamycin Signaling Pathway Contributes to Glioma Progression and Patients’ Prognosis. J Surg Res 168:97–102
Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, Woo SY, Heimberger AB, Suki D, Prados M, Chang S, Barker FG, Fuller GN, Aldape KD (2006) Prognostic Associations of Activated Mitogen-activated Protein Kinase and Akt Pathways in Glioblastoma. Clin Cancer Res 12:3935–3941
Setsu N, Kohashi K, Endo M, Yamamoto H, Tamiya S, Takahashi Y, Yamada Y, Ishii T, Matsuda S, Yokoyama R, Iwamoto Y, Oda Y (2013) Phosphorylation of Signal Transducer and Activator of Transcription 3 in Soft Tissue Leiomyosarcoma is Associated with a Better Prognosis. Int J Cancer 132:109–115
Sebestyen A, Sticz TB, Mark A, Hajdu M, Timar B, Nemes K, Nagy N, Varadi Z, Kopper L (2012) Activity and Complexes of mTOR in Diffuse large B-cell Lymphomas–a Tissue Microarray Study. Mod Pathol 25:1623–1628
Tampellini M, Longo M, Cappia S, Bacillo E, Alabiso I, Volante M, Dogliotti L, Papotti M (2007) Co-expression of EGF Receptor, TGFalpha and S6 Kinase is Significantly Associated with Colorectal Carcinomas with Distant Metastases at Diagnosis. Virchows Arch 450:321–328
Chaisuparat R, Rojanawatsirivej S, Yodsanga S (2013) Ribosomal Protein S6 Phosphorylation is Associated with Epithelial Dysplasia and Squamous Cell Carcinoma of the Oral Cavity. Pathol Oncol Res 19:189–193
Baryawno N, Sveinbjörnsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI (2010) Small-molecule Inhibitors of Phosphatidylinositol 3-Kinase/Akt Signaling Inhibit Wnt/beta-Catenin Pathway Cross-talk and Suppress Medulloblastoma Growth. Cancer Res 70:266–276
Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, Mills GB, Hung MC, Meric-Bernstam F (2004) Determinants of Rapamycin Sensitivity in Breast Cancer Cells. Clin Cancer Res 10:1013–1023
Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, García-Echeverría C (2008) Identification and Characterization of NVP-BEZ235, A New Orally Available Dual phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor with Potent in vivo Antitumor Activity. Mol Cancer Ther 7:1851–1863
Wolff JE, Brown RE, Buryanek J, Pfister S, Vats TS, Rytting ME (2012) Preliminary Experience with Personalized and Targeted Therapy for Pediatric Brain Tumors. Pediatr Blood Cancer 59:27–33
Acknowledgments
We thank Mária Csorba, Edit Parsch, Zsuzsanna Kaminszky and Renáta Kiss for excellent technical assistance and Dr. J. Silber (University of Washington, Seattle, WA, USA) for providing UW228–2 medulloblastoma cell line.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pócza, T., Sebestyén, A., Turányi, E. et al. mTOR Pathway As a Potential Target In a Subset of Human Medulloblastoma. Pathol. Oncol. Res. 20, 893–900 (2014). https://doi.org/10.1007/s12253-014-9771-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-014-9771-0