Abstract
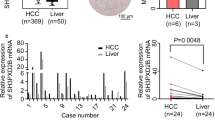
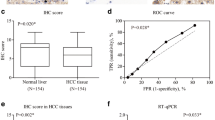
In this study,we investigated the ADAM8 expression in hepatocellular carcinoma (HCC) and its correlation with clinicopathologic features,including the survival of patients with HCC. Furthermore,we examined the biological processes regulated by ADAM8 during the development of using HepG2 cell line as a model system. We used immunohistochemistry to compare ADAM8 protein expression in HCC and normal liver tissues and further analyze the ADAM8 protein expression in clinicopathologically characterized 105 HCC cases.We stably knocked down the endogenous expression level of ADAM8 in HepG2 cells with specific shRNA-expressing lentiviral vector. Following the successful establishment of stable cells,we examined in vitro cell growth by MTT assay,anchorage-independent growth by soft-agar colony formation assay and cell migration/invasion by transwell and boyden chamber assay. And in addition,we also investigated the in vivo tumor growth by xenograft transplantation of HepG2 cells into nude mice. Protein expression level of ADAM8 was markedly higher in HCC tissues than that in the normal liver tissues (P = 0.0058).In addition,high expression of ADAM8 protein was positively correlated with serum AFP elevation,tumor size,histological differentiation,tumor recurrence,tumor metastasis,and tumor stage. Patients with higher ADAM8 expression showed a significantly shorter overall survival time than patients with low ADAM8 expression. Multivariate analysis suggested that ADAM8 expression might be an independent prognostic indicator (p = 0.016) for the survival of patients with HCC. ADAM8-specific shRNA (shADAM8) successfully knocked down its endogenous expression in HepG2 cells. Compared to the parental and control shRNA-transfected (shCtrl) HepG2 cells,the shADAM8 cells exhibited significantly reduced in vitro cell growth,anchorage-independent growth,cell migration and invasion (p < 0.05).In vivo,the xenograft transplants from shADAM8 cells gave rise to much smaller tumors as compared to those from shCtrl cells. High ADAM8 expression is associated with poor overall survival in patients with HCC. Down-regulation of ADAM8 inhibits the growth,anchorage-independent growth,migration and invasion of HepG2 cells. ADAM8 may be a potential target of antiangiogenic therapy for HCC.






Similar content being viewed by others
References
Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, TaylorRobinson SD (2009) Hepatocellular carcinoma:current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol 3:353–367
Lau WY, Lai EC (2008) Hepatocellular carcinoma:current management and recent advances. Hepatobiliary Pancreat Dis Int 7:237–257
Koorey D (2007) Hepatocellular carcinoma:prevention, detection and treatment in the real world. Intern Med J 37:513–515
Pleguezuelo M, Marelli L, Misseri M, Germani G, Calvaruso V, Xiruochakis E et al (2008) TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 8:1623–1641
Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S (1997) CD156(human ADAM8):expression, primary amino acid sequence, and gene location. Genomics 41(1):56–62
Yamamoto S, Higuchi Y, Yoshiyama K et al (1999) ADAM family proteins in the immune system. Immunol Today 20(6):278–284
Schlomann U, Wildeboer D, Webster A et al (2002) The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. Biol Chem 277(50):48210–48219
Primakoff P, Myles DG (2000) The ADAM gene family:surface proteins with adhesion and protease activity. Trends Genet 16(2):83–87
Seals DF, Courtneidge SA (2003) The ADAMs family of metalloproteases:multidomain proteins with multiple functions. Genes Dev 17(1):7–30
Fourie AM, Coles F, Moreno V, Karlsson L (2003) Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem 278(33):30469–30477
Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW (2004) Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem 279(16):16083–16090
Karan D, Lin FC, Bryan M et al (2003) Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int J Oncol 23(5):1365–1371
O’Shea C, McKie N, Buggy Y et al (2003) Expression of ADAM-9 mRNA and protein in human breast cancer. Int J Cancer 105(6):754–761
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ et al (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26:2707–2716
Valkovskaya N, Kayed H, Felix K, Hartmann D, Giese NA, Osinsky SP, Friess H, Kleeff J (2007) ADAM8 expression is associated with increased invasiveness and reduced patient survival in pancreatic cancer. J Cell Mol Med 11(5):1162–1174
Valkovskaya NV (2008) Hypoxia-dependent expression of ADAM8 in human pancreatic cancer cell lines. Exp Oncol 30(2):129–132
Kelly K, Crowley J, Bunn PA Jr et al (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer:a Southwest Oncology Group trial. J Clin Oncol 19(13):3210–3218
Hennessy BT, Hanrahan EO, Daly PA (2004) Non-Hodgkin lymphoma:an update. Lancet Oncol 5(6):341–353
Pujol JL, Grenier J, Daures JP, Daver A, Pujol H, Michel FB (1993) Serum fragment of cytokeratin subunit 19 measured by CYFRA 21–1 immunoradiometric assay as a marker of lung cancer. Cancer Res 53(1):61–66
Miyake Y, Kodama T, Yamaguchi K (1994) Pro-gastrin-releasing peptide(31–98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res 54(8):2136–2140
Michael M, Babic B, Khokha R et al (1999) Expression and prognostic significance of metalloproteinases and their tissue inhibitors in patients with small-cell lung cancer. J Clin Oncol 17(6):1802–1808
Peng SY, Lai PL, Chu JS et al (1993) Expression and hypomethylation of alpha-fetoprotein gene in unicentric and multicentric human hepatocellular carcinomas. Hepatology 17:35–41
Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY (2000) b-Catenin mutations are associated with a subset of low stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 157:763–770
Tuck AB, O'Malley FP, Singhal H et al (1998) Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer 79:502–508
Ue T, Yokozaki H, Kitadai Y et al (1998) Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer 79:127–132
Hwang YH, Choi JY, Kim S et al (2004) Over-expression of c-raf-1proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol Res 29(2):113–121
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Tan, YF., Jiang, C. et al. High ADAM8 Expression is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Pathol. Oncol. Res. 19, 79–88 (2013). https://doi.org/10.1007/s12253-012-9560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-012-9560-6