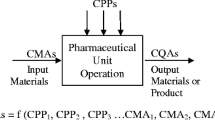
Abstract
Development of the arzoxifene hydrochloride drug substance manufacturing process, first via a traditional approach and subsequently via an enhanced approach, provides an informative case study in quality by design (QbD). The primary focus of this paper is to illustrate the impact and advantages of QbD on the impurity control strategy. By operating the process at the extremes during design space studies, a larger collection of organic impurities and higher levels of typical impurities are observed in the intermediates. This enables a more thorough understanding of the ability of the process to purify the drug substance and results in a more complete and robust set of intermediate specifications as well as broader and more robust analytical methods. We demonstrate that, when each of the synthetic steps are operated within the design space, the byproduct impurities in the intermediates will not exceed levels found to be rejected in subsequent steps, ensuring that the drug substance will meet its critical quality attributes. Through the rigorous application of an enhanced process development approach, we have designed quality into the arzoxifene hydrochloride drug substance. As a result, real-time release of the intermediate batches is proposed to increase the process throughput and avoid the expense of nonvalue-added testing.















Similar content being viewed by others
References
Effects of arzoxifene on bone fractures and incidence of breast cancer. Study NCT00088101 (H4Z-MC-GJAD). http://www.clinicaltrials.gov/ct2/show/NCT00088010?term=arzoxifene&rank=1.
Eli Lilly and Company. Press release. 18 August 2009. http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed 24 Aug 2009.
Olsen BA, Baertschi SW. Strategies for investigation and control of process- and degradation-related impurities. Sep Sci Technol. 2004;5:89–117.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use. Pharmaceutical Development Q8(R2). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use. Quality Risk Management (Q9). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use. Pharmaceutical Quality System (Q10). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use. Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) (Q11). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf.
Burt JL, Braem AD, Ramirex A, Mudryk B, Rossano L, Tummala S. Model-guided design space development for a drug substance manufacturing process. J Pharm Innov. 2011;6:181–92.
Cimarosti Z, Bravo F, Castoldi D, Tinazzi F, Provera S, Perboni A, et al. Application of the QbD principles in the development of the Casopitant mesylate manufacturing process. Research studies for the definition of the control strategy of some drug substance-CQAs for stages 2a, 2b, and 2c. Org Process Res Dev. 2010;14:805–14.
Cimarosti Z, Bravo F, Stonestreet P, Tinazzi F, Vecchi O, Camurri G. Application of quality by design principles to support development of a control strategy for the control of genotoxic impurities in the manufacturing process of a drug substance. Org Process Res Dev. 2010;14:993–8.
Castagnoli C, Yahyah M, Cimarosti Z, Peterson JJ. Application of quality by design principles for the definition of a robust crystallization process for casopitant mesylate. Org Process Res Dev. 2010;14:1407–19.
am Ende D, Bronk K, Mustakis J, O'Connor G, Santa Maria C, Nosal R, et al. API quality by design example from the Torcetrapib manufacturing process. J Pharm Innov. 2007;2:71–86.
Alt C, Kjell D, Zhang T, Zhang F, Fennell J, Seibert K. United States patent application publication. US 2010/0137602 A1, 3 June 2010.
Dotterer S, Forbes R, Hammill C. Impact of metal-induced degradation on the determination of pharmaceutical compound purity and a strategy for mitigation. J Pharm Biomed Anal. 2011;54:987–94.
Seibert KD, Sethuraman S, Mitchell JD, Griffiths KL, McGarvey B. The use of routine process capability for the determination of process parameter criticality in small-molecule API synthesis. J Pharm Innov. 2008;3:105–12.
am Ende D, Seymour C, Watson T. A science and risk based proposal for understanding scale and equipment dependencies of small molecule drug substance manufacturing processes. J Pharm Innov. 2010;5:72–8.
Acknowledgments
The authors would like to acknowledge Kevin Seibert for his leadership in the application of QbD to the arzoxifene process. Phil Hoffman and Odilon Campos are also acknowledged for their contributions to the PAR, DS, and bracketing studies. Doug Kjell is acknowledged for his leadership in developing the synthetic route. Guy Risedorph, Matt Earley, Tim Aldridge, and Mary Kay McCauley are acknowledged as members of the analytical team, participating in method development and control strategy development. Jacek Wanczura and Vickie Horsley are acknowledged for providing sample analysis supporting this work. Eric Jensen is acknowledged for review of this manuscript and providing many helpful corrections and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castle, B.C., Forbes, R.A. Impact of Quality by Design in Process Development on the Analytical Control Strategy for a Small-Molecule Drug Substance. J Pharm Innov 8, 247–264 (2013). https://doi.org/10.1007/s12247-013-9165-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-013-9165-y