Abstract
In the last decade, efforts to reconstruct suprageneric phylogeny of the Cyperaceae have intensified. We present an analysis of 262 taxa representing 93 genera in 15 tribes, sequenced for the plastid rbcL and trnL-F (intron and intergenic spacer). Cyperaceae are monophyletic and resolved into two clades, here recognised as Mapanioideae and Cyperoideae, and the overall topology is similar to results from previous studies. Within Cyperoideae, Trilepideae are sister to rest of taxa whereas Cryptangieae, Bisboeckelerieae and Sclerieae are resolved within Schoeneae. Cladium and Rhynchospora (and Pleurostachys) are resolved into clades sister to the rest of Schoeneae, lending support to the recognition of these taxa in separate tribes. However, we retain these taxa in Schoeneae pending broader sampling of the group. The phylogenetic position of 40 species in 21 genera is presented in this study for the first time, elucidating their position in Abildgaardieae (Trachystylis), Cryptangieae (Didymiandrum, Exochogyne), Cypereae (Androtrichum, Volkiella), Eleocharideae (Chillania), and Schoeneae (Calyptrocarya, Morelotia). More sampling effort (more taxa and the use of more rapidly evolving markers) is needed to resolve relationships in Fuireneae and Schoeneae.
Similar content being viewed by others
Introduction
Cyperaceae comprise 109 genera and approximately 5,500 species and have an almost cosmopolitan distribution (Govaerts et al., 2007). About 35% of the genera are monotypic, 26% have two to five species, and there are a seven (6%) genera with over 200 species, the largest being Cyperus (686 species) and Carex (1,757 species; Goetghebeur, 1998). The family shows extreme reduction in floral morphology, and the majority of the smaller genera are carved out of the larger ones on the basis on one or few distinguishing features.
Family level phylogenetic studies in the last ten years have used morphological (e.g. Simpson, 1995; Bruhl, 1995; Goetghebeur, 1998), molecular (e.g. Muasya et al., 1998; Simpson et al., in press), and combined morphological and molecular data (Muasya et al., 2000b). The two most recent classifications based on morphological data (including gross morphology, anatomy and embryology) differ in suprageneric groupings of tribes and subfamilies. Bruhl (1995) recognised two subfamilies, Cyperoideae and Caricoideae, whereas Goetghebeur (1998) recognised two additional subfamilies, Sclerioideae and Mapanioideae, both of which were included in Caricoideae by Bruhl (1995). The treatments also differed in tribal circumscription, with Bruhl (1995) recognising 12 tribes and treating Scirpeae broadly to include taxa classified in tribes Dulicheae, Fuireneae, Eleocharideae and Cypereae sensu Goetghebeur (1998).
Molecular DNA sequence data are increasingly used in angiosperm classification. In Cyperaceae, broad suprageneric studies have so far sampled all subfamilies and tribes, but sampling effort is not evenly distributed among all tribes. Family-level studies have been based mainly on rbcL sequence data (e.g. Muasya et al., 1998; Simpson et al., 2007), whereas at tribal or subfamilial levels other plastid and nuclear regions have been used. The plastid regions rps16 intron, trnL intron and trnL-F intergenic spacer have been used in studies of subfamily Mapanioideae (e.g. Simpson et al., 2003) and a number of studies at tribal and generic level.
This study uses three of the most commonly used plastid regions (the rbcL gene, the trnL intron, and the trnL-F spacer) to reconstruct relationships of the family and presents an overview of the current status of suprageneric phylogenetic studies. The rbcL gene has been sequenced for over 60% genera of Cyperaceae (e.g. Simpson et al., 2007) and can be aligned unambiguously, whereas trnL-F (both the trnL intron and the trnl-F intergenic spacer) has been used to a greater extent in generic studies and is more difficult to align at the family level.
Analysis of rbcL and trnl-F Data
The analysis includes a total 262 taxa (258 species) of Cyperaceae in 93 genera from the 15 tribes and four subfamilies recognised by Goetghebeur (1998). Sequences from previous studies (Bremer, 2002; Dhooge et al., 2003; Muasya et al., 1998, 2000a, 2000b, 2001, 2002; Simpson et al., 2003, 2007; Verboom, 2006; Zhang et al., 2004) were analysed together with 41 newly sequenced taxa representing 22 genera, nine of which had not been previously sequenced. Total DNA was extracted from vegetative material (leaves or culms) collected in the field or from herbarium specimens (Table 1). DNA extraction, amplification and sequencing were performed according to published procedures (e.g. Muasya et al., 2002); the resulting sequences were aligned manually and are lodged with GenBank (Table 1).
Heuristic analyses were carried out using PAUP* (Swofford, 2002). Searches were conducted under Fitch (1971) parsimony, TBR (tree-bissection-reconnection) branch swapping, and random taxon addition (5,000) with the MulTrees option in effect and retaining only ten trees per replicate. Internal support was estimated using 1000 bootstrap replicates (Felsenstein, 1985), with the following search parameters: simple taxon addition, TBR branch-swapping, and MulTrees option in effect with only ten trees held per step.
The aligned matrix has 3,573 characters comprising 1,428 from rbcL and 2145 from trnl-F (intron and intergenic spacer) region. Some portions of trnl-F could not be unambiguously aligned, and 865 characters were excluded from the analysis, leaving 2,708 characters, of which 913 are potentially parsimony informative.
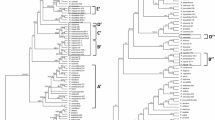
Fifty equally parsimonious trees were recovered of length = 5,467 steps, consistency index (CI) = 0.45 and retention index (RI) = 0.79. The strict consensus tree (Figs. 1, 2 and 3) is presented and discussed below.
Maximum parsimony strict consensus tree of Cyperaceae, showing the outgroup and Cyperaceae tribes Hypolytreae (Hy), Chrysitricheae (Ch), Trilepideae (Tr), Schoeneae (Sc), Sclerieae (Scl), Bisboeckelereae (Bi) and Cryptangieae. Goetghebeur’s (1998) classification and the proposed classification are marked by grey and black bars respectively. Bootstrap support values shown as weak (* = 50–74%), moderate (** = 75–89%) and strong (*** = 90–100%)
Maximum parsimony strict consensus tree of Cyperaceae, showing the Cyperaceae tribes Schoeneae (Sc), Cariceae, Scirpeae (S), Dulicheae (Du), Eleocharideae (El), Fuireneae (Fu), Abildgaardieae (Ab), Arthrostylideae (Ar), and Cypereae (Cy). Goetghebeur’s (1998) classification and the proposed classification are marked by black grey and black respectively. Bootstrap support values shown as weak (* = 50–74%), moderate (** = 75–89%) and strong (*** = 90–100%)
Maximum parsimony strict consensus tree of Cyperaceae, showing the Cyperaceae tribes Cypereae (Cy), Scirpeae (S) and Chrysitricheae (Ch). Goetghebeur’s (1998) classification and the proposed classification are marked by grey and black bars respectively. Bootstrap support values shown as weak (* = 50–74%), moderate (** = 75–89%) and strong (*** = 90–100%)
Subfamily Relationships
Cyperaceae are resolved as monophyletic and sister to Juncaceae, with Mapanioideae sister to all the other Cyperaceae (Fig. 1; tribes and subfamilies sensu Goetghebeur, 1998). Within the last clade, Coleochloa-Microdracoides (Trilepideae) form a clade sister to the rest of Cyperaceae. Trilepideae are not sister to Scleria-Diplacrum (Sclerieae) as the latter are embedded in the Schoeneae, and therefore the Sclerioideae are not monophyletic. Also Caricoideae are sister to Scirpeae and embedded within Cyperoideae (Fig. 2).
Similar studies in which Mapanioideae are resolved as sister to rest of Cyperaceae have been reported by Bruhl (1995) based on morphological studies and in previous family-level DNA studies (e.g. Muasya et al., 1998, 2000b; Simpson et al., 2007). Mapanioideae have a unique floral morphology compared with the rest of Cyperaceae, with floral units each comprising two to ten or more scales (the lower ones being keeled), two to ten stamens and a single gynoecium. The floral units have been variously interpreted as bisexual flowers in which the arrangement of the structures has been disturbed (Goetghebeur, 1998) or as reduced partial inflorescences termed spicoids (Simpson et al., 2003; Richards et al., 2006).
Recognition of Caricoideae and Sclerioideae as subfamilies separate from Cyperoideae (e.g. Goetghebeur, 1998), based on unique morphological characters is not supported by current analysis. Typical Cyperoideae are mostly diagnosed by having at least one (sometimes all) bisexual flower, whereas in Sclerioideae (and some unusual Cyperoideae) they are all unisexual and in Caricoideae they are all unisexual and enclosed by a utricle. This study and other analyses of DNA data support the recognition of two subfamilies in Cyperaceae, Mapanioideae and Cyperoideae, as proposed by Simpson et al. (2003, 2007).
Tribes of the Cyperaceae
A number of tribal groups recognised in the recent classification of Cyperaceae by Goetghebeur (1998) are supported by the current study. Within Mapanioideae, some Hypolytreae (Mapania, Hypolytrum and Scirpodendron) and Chrysitricheae (Lepironia, Chrysitrix and Chorizandra) form clades separate from a polytomy comprising other mapanioids (Fig. 1). Although the polytomy observed may be caused by insufficient data for some of the taxa, Capitularina and Exocarya (both of which traditionally have been placed in Hypolytreae) were resolved together in Chrysitricheae in a combined pollen and DNA data study (Simpson et al., 2003).
The inselberg taxa in Trilepideae (Coleochloa to Microdracoides; Fig. 1) form a strongly supported clade. This clade is sister to the rest of Cyperoideae and not to other tribes of Sclerioideae (sensu Goetghebeur 1998), namely Cryptangieae, Sclerieae and Bisboeckelereae. These other tribes are embedded among Schoeneae (Fig. 1). Notable is the position of Exochogyne, a genus unplaced in any tribe of Sclerioideae by Goetghebeur (1998) due to unclear morphological homologies, and which is resolved here among Cryptangieae.
Schoeneae are one of the most heterogeneous tribes in the family, having 29 genera of which Rhynchospora is among the largest; over 50% of the genera have fewer than 10 species (Goetghebeur, 1998). This analysis resolves four clades within Schoeneae: (1) Cladium, (2) Gymnoschoenus, (3) Caustis to Didymiandrum, and (4) Rhynchospora (Figs. 1 and 2). The moderately supported Rhynchospora clade has been previously classified in a separate tribe Rhynchosporeae (e.g. Goetghebeur, 1986; Bruhl, 1995) on the basis of, inter alia, distinct style base. Members of the former Sclerioideae (Cryptangieae, Bisboeckelereae and Sclerieae) are resolved among clade (3), an observation reported in previous studies (e.g. Simpson et al., 2007). Schoeneae have an essentially Gondwanan distribution, and several widely distributed genera (e.g. Costularia, Tetraria; Zhang et al., 2004, Verboom, 2006) are polyphyletic. Morelotia is resolved in a clade which includes Costularia, Tricostularia and reticulate-sheathed Tetraria (Tricostularia clade in Verboom, 2006), and not together with Ghania. A close relationship between Morelotia and Ghania has been suggested by several authors (e.g. Goetghebeur, 1986), while Bruhl (1995) argued against this relationship after recovering Morelotia distant from Ghania. The monotypic Schoenoides (Seberg, 1988) is embedded in Oreobolus here and in other studies (Mandriñán et al., 2004), further supporting the inclusion of Schoenoides in Oreobolus (e.g. Curtis & Morris, 1994; Govaerts et al., 2007). There have been limited phylogenetic studies in Schoeneae (e.g. Zhang et al., 2004; Verboom, 2006), which lack bootstrap support for the basal nodes, and more data are needed to resolve relationships among the taxa. Further studies of Schoeneae are in progress (Bruhl et al. and Verboom et al., unpublished data).
The moderately supported clade (Khaosokia to Dulichium) includes members of Cariceae, Scirpeae and Dulicheae (Fig. 2). Khaosokia is resolved sister to the rest of the members of this clade, a position suggested by Simpson et al. (2005) from observations of gross morphology and DNA studies. Scirpeae are not monophyletic, as Dulicheae are embedded between Scirpeae I and Scirpeae II. In Scirpeae I, the generic boundaries between Trichophorum and Oreobolopsis are unclear, and further attention is needed to resolve the polyphyly of Trichophorum. Phylogenetic studies involving Andean species of Scirpus have recently led to description of a new segregate genus, Zameioscirpus (e.g. Dhooge et al., 2003). Carex is polyphyletic and includes other genera of Cariceae, a similar pattern has been observed in previous studies (e.g. Yen & Olmstead, 2000; Starr et al., 2004)
Fuireneae are split into four clades (Fig. 2) in our analysis. Fuireneae I (Fuirena) is sister to Eleocharideae, Fuireneae II (Bolboschoenus) is sister to Abildgaardieae, whereas Fuireneae III (Schoenoplectus and Actinoschoenus) and Fuireneae IV (Schoenoplectiella group) form a polytomy with Cypereae. Relationships among these groups based on DNA data remain unstable (cf. Simpson et al., 2007). Schoenoplectus is paraphyletic with several tropical African perennial taxa (e.g. S. mucronatus) being resolved together with Schoenoplectiella. Schoenoplectiella, recently segregated to include annual amphicarpous taxa of Schoenoplectus (Lye, 2003), is resolved into a strongly supported clade that includes perennial tropical Schoenoplectus species sharing a lateral spikelet morphology. Further studies are in progress (Muasya et al., unpublished data) evaluating relationships in the group.
Abildgaardieae are resolved to include Arthrostylis aphylla, Trachystylis strandbrokensis and Actinoschoenus repens (Fig. 2), taxa which have been previously placed in Schoeneae (Goetghebeur, 1998). Arthrostylis and Actinoschoenus have been shown to be closer to Abildgaardieae based on plastic and nuclear ribosomal (ITS) data (Ghamkhar et al., 2007). Both Arthrostylis and Trachystylis are monotypic Australian taxa with bisexual flowers that lack perianth segments, but share gross morphological similarity with Schoeneae (e.g. one- to few-flowered spikelet and wide glume wings enclosing the next flower). On the other hand, Actinoschoenus repens is a Zambian endemic, with morphological similarity to both Abildgaardieae and Schoeneae. Although these three taxa had been placed in Schoeneae even with decisive anatomical and embryological data lacking, the DNA data resolve them in Abildgaardieae, and similar results were obtained independently by Ghamkhar et al. (2007). We therefore propose their formal inclusion in this tribe.
Cypereae form a strongly supported clade (Fig. 3) that has received intensive DNA phylogenetic study, both at generic (e.g. Muasya et al., 2001, 2002) and tribal levels (Muasya et al., 2008). Cypereae are characterised by the presence of Cyperus-type embryo and here include Hellmuthia, a genus previously considered to belong in Chrysitricheae (e.g. Haines & Lye, 1976; Goetghebeur, 1998; cf. Vrijdaghs et al., 2006, 2008). Scirpus falsus and S. ficinioides, taxa from the Drakensberg Mountians in South Africa and previously placed in Scirpeae, are resolved here among Cypereae in a clade including Ficinia, Isolepis, Hellmuthia and Scirpoides. More studies are in progress to describe a new genus including these taxa (authors, unpublished data).
Revised Suprageneric Classification of Cyperaceae
Based on the available data, we support the revised classification of Cyperaceae into two subfamilies, Mapanioideae and Cyperoideae (Figs. 1, 2 and 3). We also broadly accept the tribal circumscriptions of Goetghebeur (1998) but with modification to tribes Cypereae (to include Hellmuthia and the perianth-bearing Drakensberg Scirpus, S. falsus and S. ficinioides); Abildgaardieae (to include Arthrostylis, Trachystylis and Actinoschoenus); Schoeneae (recognising Rhynchosporeae, Rhynchospora and Pleurostachys); and Cryptangieae (to include Didymiandrum and Exochogyne). We refrain from recognising Cladieae (Cladium) pending more studies.
Future Research
Choice of marker and uneven sampling limit the scope for analysing different data sets in combination. The current study and a number of other ongoing studies have focused on more slowly evolving plastid regions, which have less resolution but can be aligned across the family. Among research groups in different institutes, there is need to study the same DNA regions (e.g. rbcL, trnl-F, rps16) for similar taxa to enable different data sets to be aligned in combination.
The intensity of sampling varies among tribes. Although Chrysitricheae, Cypereae, Hypolytreae, Scirpeae and Cariceae are among the better studied tribes, more effort is needed to elucidate phylogenetic relationships within Cryptangineae, Bisboeckelerieae, Fuireneae, Schoeneae, and Sclerieae.
Literature cited
Bremer, K. (2002). Gondwanan evolution of the grass alliance of families (Poales). Evolution 56: 1374–1387.
Bruhl, J. J. (1995). Sedge genera of the world: Relationships and a new classification of the Cyperaceae. Austral. Syst. Bot. 8: 125–305.
Chase, M. W., D. E. Soltis, R. G. Olmstead, D. Morgan, D. H. Les, B. D. Mishler, M. R. Duvall, R. A. Price, H. G. Hills, Y. -L. Qiu, K. A. Kron, J. H. Rettig, E. Conti, J, D. Palmer, J. R. Manhart, K. J. Sytsma, H. J. Michaels, W. J. Kress, K. G. Karol, W. D. Clark, M. Hedrén, B. S. Gaut, R. K. Jansen, K. -J. Kim, C. F. Wimpee, J. F. Smith, G. R. Furnier, S. H. Strauss, Q. -Y. Xiang, G. M. Plunkett, P. S. Soltis, S. M. Swenson, S. E. Williams, P. A. Gadek, C. J. Quinn, L. E. Eguiarte, E. Golenberg, G. H. Learn Jr., S. W. Graham, S. C. H. Barrett, S. Dayanandan, & V. A. Albert. (1993). Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Ann. Missouri Bot. Gard. 80: 528–586.
Curtis, W. M. & D. M. Morris. (1994). The Student’s Flora of Tasmania. Part 4b. Angiospermae: Alismataceae to Burmanniaceae. Hobart: St. David’s Park Publishing
Dhooge, S., P. Goetghebeur & A. M. Muasya. (2003). Zameioscirpus, a new genus of Cyperaceae from South America. Plant Syst. Evol. 2431–2: 73–84
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–783.
Fitch, W. M. (1971). Toward defining the course of evolution: Minimum change for a specific tree topology. Syst. Zool. 20: 406.
Ghamkhar, K., A. D. Marchant, K. L. Wilson & J. J Bruhl. (2007). Phylogeny of Abildgaardieae (Cyperaceae) inferred from ITS and trnl-F data. Aliso 23: 149–164.
Goetghebeur, P. (1986). Genera Cyperacearum. Dr. Sci. thesis, State University, Ghent
————— (1998). Cyperaceae. In: K. Kubitzki, The families and genera of vascular plants 4: 164. Springer, Berlin.
Govaerts, R., D. A. Simpson, P. Goetghebeur, K. L. Wilson, T. Egorova, & J. Bruhl. (2007). World checklist of Cyperaceae. The Board of Trustees of the Royal Botanic Gardens, Kew.
Haines, R. W. & K. A. Lye. (1976). Studies in African Cyperaceae 14. The genus Hellmuthia Steud. Bot. Notiser 129: 61–67.
Kress, W. J., K. J. Wurdack, E. A. Zimmer & D. H. Janzen. (2005). Use of DNA barcodes to identify flowering plants. PNAS 102: 8369–8374.
Lye, K. A. (2003). Schoenoplectiella Lye, gen. nov. (Cyperaceae). Lidia 6: 20–29.
Mandriñán, S., J. Chacón, & M. W. Chase. (2004). Molecular phylogenetics and historical biogeography of Oreobolus (Cyperaceae). Cladistics 20: 88.
Muasya, A. M., D. A. Simpson, M. W. Chase, & A. Culham. (1998). An assessment of the suprageneric phylogeny in Cyperaceae using rbcL DNA sequences. Plant Syst. Evol. 211: 257–271.
————, ————, ————, & ————. (2000a). Phylogenetic relationships within the heterogeneous Scirpus sensu lato. (Cyperaceae) inferred from rbcL and trnl-F sequence data. In K. L. Wilson & D. A. Morrison (eds.), Monocots: Systematics and Evolution, pp. 610–614. CSIRO, Melbourne.
Muasya, A. M., J. J. Bruhl, D. A. Simpson, A. Culham, & M. W. Chase. (2000b). Suprageneric phylogeny of Cyperaceae: A combined analysis. In K. L. Wilson & D. A. Morrison, [eds.], Monocots: Systematics and Evolution, pp. 593–601. CSIRO, Melbourne, Victoria, Australia.
————, ————, ————, & ———— (2001). A phylogeny of Isolepis (Cyperaceae) inferred using plastid rbcL and trnl-F sequence data. Syst. Bot. 26: 342–353.
————, D. A. Simpson, & M. W. Chase. (2002). Phylogenetic relationships in Cyperus s.l. (Cyperaceae) inferred from plastid DNA sequence data. Bot. J. Linn. Soc. 138: 145–153.
————, A. Vrijdaghs, D. A. Simpson, M. W. Chase, P. Goetghebeur & E. Smets. (2008). What is a genus in Cypereae: phylogeny, character homology assessment and generic circumscription. Bot. Rev. (this volume).
Plunkett, G. M., D. E. Soltis, P. S. Soltis & R. E. Brooks. (1995). Phylogenetic relationships between Juncaceae and Cyperaceae based on rbcL sequence data. Amer. J. Bot. 82: 520–525.
Richards, J. H., J. J. Bruhl & K. L. Wilson. (2006). Flower or spikelet?—Understanding the morphology and development of reproductive structures in Exocarya (Cyperaceae, Mapanioideae, Chrysitricheae). American Journal of Botany 93: 1241–1250.
Roalson, E. H., J. T. Columbus & E. A. Friar. (2001). Phylogenetic relationships in cariceae (Cyperaceae) based on ITS (nrDNA) and trnT-LF (cpDNA) region sequences: Assessment of subgeneric and sectional relationships in Carex with emphasis on section Acrocystis. Syst. Bot. 26: 318–341.
Seberg, O. (1988). Taxonomy, phylogeny and biogeography of the genus Oreobolus R. Br. (Cyperaceae), with comments on the biogeography of the South Pacific continents. Bot. J. Linn. Soc. 96: 119–195.
Simpson, D. A. (1995). Relationships with Cyperales. In P.J. Rudall, P.J. Cribb, D.F. Cutler, C.J. Humpries (eds.), Monocotyledons: Systematics and Evolution, 497–509. Royal Botanic Gardens, Kew, Richmond.
Simpson, D. A., C. A. Furness, T. R. Hodkinson, A. M. Muasya & M. W. Chase. (2003). Phylogenetic relationships in Cyperaceae subfamily Mapanioideae inferred from pollen and plastid DNA sequence data. Am. J. Bot. 90: 1071–1086.
————, A. M. Muasya, K. Chayamarit, J. A. N. Parnell, S. Suddee, B. D. E. Wilde, M. B. Jones, J. J. Bruhl, & R. Pooma. (2005). Khaosokia caricoides, a new genus and species of Cyperaceae from Thailand. Bot. J. Linn. Soc. 149: 357–364.
————, ————, M. Alves, J. J Bruhl, S. Dhooge, M. W. Chase, C. A. Furness, K. Ghamkhar, P. Goetghebeur, T. R. Hodkinson, A. D. Marchant, R. Nieuborg, A. A. Reznicek, E. H. Roalson, E. Smets, J. R. Starr, W. W. Thomas, K. L. Wilson & X. Zhang. (2007). Phylogeny of Cyperaceae based on DNA sequence data—A new rbcL analysis. Aliso 23: 72–83.
Starr, J. R., S. A. Harris, & D. A. Simpson. (2004). Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae I: Generic relationships and evolutionary scenarios. Syst. Bot. 29: 528–544.
Swofford, D. L. (2002). PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), version 4. Sinauer, Sunderland, MA, USA.
Verboom, G. A. (2006). A phylogeny of the schoenoid sedges (Cyperaceae: Schoeneae) based on plastid DNA sequences, with special reference to the genera found in Africa. Mol. Phylogenet. Evol. 38: 79–89.
Vrijdahs, A., P. Goetghebeur, E. Smets & A. M. Muasya. (2006). The floral scales in Hellmuthia (Cyperaceae, Cyperoideae) and Paramapania (Cyperaceae, Mapanioideae), an ontogenetic study. Ann Bot 98: 619–630.
————, A. M. Muasya, P. Goetghebeur, P. Caris, A. Nagels & E. Smets. (2008). A floral ontogenetic approach questions of homology within the Cyperoideae (Cyperaceae). Botanical Review (this volume).
Wardle, P., C. Ezcurra, C. Ramirez & S. Wagstaff. (2001). Comparison of the Xora and vegetation of the southern Andes and N. Z. J. Bot. 39: 69–108.
World Checklist of Monocotyledons (2006). The Board of Trustees of the Royal Botanic Gardens, Kew. Published on the Internet; http://www.kew.org/wcsp/monocots/ accessed 30 March 2006; 13:00 GMT.
Yen, A. C., & R. G. Olmstead. (2000). Molecular systematics of Cyperaceae tribe Cariceae based on two chloroplast DNA regions: ndhF and trnL intron-intergenic spacer. Syst. Bot. 25: 479–494.
Zhang, X., A. Marchant, K. L. Wilson, & J. J. Bruhl. (2004). Phylogenetic relationships of Carpha and its relatives (Schoeneae: Cyperaceae) inferred from chloroplast trnL intron and trnL-trnF intergenic spacer sequences. Mol. Phylogenet. Evol. 31: 647–657.
Acknowledgements
A number of the contributors acknowledge funding from various sources. AMM acknowledges funding from the Belgian Fund for Scientific Research-Flanders (FWO-Vlaanderen, G.0104.01N); K.U.Leuven (grant F/02/052); University of Cape Town (Smuts Fellowship); and the Norwegian Council of Universities’ Committee for Development Research and Education (NUFU project 53/03). GAV acknowledges funding from the National Research Council, South Africa.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Muasya, A.M., Simpson, D.A., Verboom, G.A. et al. Phylogeny of Cyperaceae Based on DNA Sequence Data: Current Progress and Future Prospects. Bot. Rev 75, 2–21 (2009). https://doi.org/10.1007/s12229-008-9019-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-008-9019-3