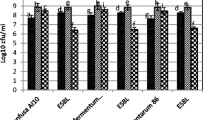
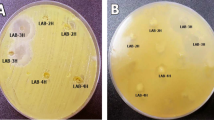
Abstract
Antimicrobial and immunomodulatory potential of various Lactobacillus reuteri strains is closely connected to their metabolite production profile under given cultivation conditions. We determined the in vitro production of antimicrobial substances such as organic acids, ethanol, and reuterin by four strains of L. reuteri (L. reuteri E, L. reuteri KO5, L. reuteri CCM 3625, and L. reuteri ATCC 55730). All studied L. reuteri strains showed the ability to produce lactic acid, acetic acid, and ethanol with concominant consumption of glucose and together with phenyllactic acid—a potent antifungal compound—with concominant consumption of phenylalanine. The reuterin production from glycerol was confirmed for all analyzed lactobacilli strains except L. reuteri CCM 3625. Production of organic acids, ethanol, and reuterin is significantly involved in antimicrobial activity of lactobacilli which was determined using the dual-culture overlay diffusion method against six indicator bacteria and five indicator moulds. In comparison to the referential L. reuteri ATCC 55730, the highest inhibition potential was observed against Escherichia coli CCM 3988 and Pseudomonas aeruginosa CCM 3955. Among analyzed indicators of moulds, the growth of Alternaria alternata CCM F-128 was the most inhibited by all four analyzed L. reuteri strains. Finally, the immunomodulatory potential of analyzed lactobacilli were proven by the determination of the in vitro production of biogenic amines histamine and tyramine. L. reuteri CCM 3625 was able to produce tyramine, and L. reuteri E and L. reuteri KO5 were able to produce histamine under given cultivation conditions.







Similar content being viewed by others
References
Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Vnema K (2010) Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 1801:1175–1183. doi:10.1016/j.bbalip.2010.07.007
Ammor S, Tauveron G, Dufour E, Chevallier I (2006) Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility 1—screening and characterization of the antibacterial compounds. Food Control 17:454–461. doi:10.1016/j.foodcont.2005.02.006
Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S (2001) Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379. doi:10.1067/mob.2001.115867
Årsköld E, Lohmeier-Vogel E, Cao R, Roos S, Rådström P, van Niel EW (2008) Phosphoketolase pathway dominates in Lactobacillus reuteri ATCC 55730 containing dual pathways for glycolysis. J Bacteriol 190:206–212. doi:10.1128/JB.01227-07
Ascar A, Treptow H (1986) Biogene amine in Lebensmitteln. Verlag Eugen Ulmer, Stuttgart, p 23
Axel C, Brosnan B, Zannini E, Peyer LC, Furey A, Coffey A, Arendt EK (2016) Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl Microbiol Biotechnol 100:1701–1711. doi:10.1007/s00253-015-7051-x
Babusyte A, Kotthoff M, Fiedler J, Krautwurst D (2013) Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J Leukoc Biol 93:1–8. doi:10.1189/jlb.0912433
Bergmeyer HU, Gawehn K (1974) Methods of enzymatic analysis. Vol 4, 2nd edn. Verlag Chemie GmbH, Weinheim
Bilková A, Kiňová Sepová H, Bilka F, Bukovský M, Balažová A, Bezáková L (2008) Identification of newly isolated lactobacilli from stomach mucus of lamb. Acta Facult Pharm Univ Comenianae 55:64–72
Bilková A, Kiňová Sepová H, Bukovský M, Bezáková L (2011) Antibacterial potential of lactobacilli isolated from a lamb. Veterinárni Medicína 56:319–324
Charlier C, Cretenet M, Even S, Le Loir Y (2008) Interactions between Staphylococcus aureus and lactic acid bacteria: an old story with new perspectives. Int J Food Microbiol 131:1–10. doi:10.1016/j.ijfoodmicro.2008.06.032
Chung TC, Axelsson L, Lindgren SE, Dobrogosz WJ (1989) In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb Ecol Health D 2:137–144
Cleusix V, Lacroix C, Vollenweider S, Duboux M, Le Blay G (2007) Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol 7:101–109. doi:10.1186/1471-2180-7-101
Cortés-Zavaleta O, López-Malo A, Hernández-Mendoza A, García HS (2014) Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int J Food Microbiol 173:30–35. doi:10.1016/j.ijfoodmicro.2013.12.016
Dalié DKD, Deschamps AM, Richard-Forget F (2010) Lactic acid bacteria—potential for control of mould growth and mycotoxins: a review. Food Control 21:370–380. doi:10.1016/j.foodcont.2009.07.011
DeRuiter J (2001) Histamine and antihistaminic agents. Principles of Drug Action; Fall 2:1–8
El-Ziney MG, Arneborg N, Uyttendaele M, Debevere J, Jacobsen M (1998) Characterization of growth and metabolite production of Lactobacillus reuteri during glucose/glycerol cofermentation in batch and continuous cultures. Biotechnol Lett 20:913–916. doi:10.1023/A:1005483215378
FAO/WHO (2014) Public health risks of histamine and other biogenic amines from fish and fishery products: Joint FAO/WHO expert meeting report In: World Health Organisation. FAO headquarters, Rome Italy, 23–27 July, 2012: © WHO 2014. http://www.who.int/foodsafety/publications/histamine_risk/en/
Gerez CL, Torino MI, Rollán G, Font de Valdez G (2009) Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 20:144–148. doi:10.1016/j.foodcont.2008.03.005
Ghanbari M, Jami M, Domig KJ, Kneifel W (2013) Seafood biopreservation by lactic acid bacteria—a review. LWT Food Sci Technol 54:315–324. doi:10.1016/j.lwt.2013.05
Gong X, Qi N, Wang X, Lin L, Li J (2014) Ultra-performance convergence chromatography (UPC2) method for the analysis of biogenic amines in fermented foods. Food Chem 162:172–175. doi:10.1016/j.foodchem.2014.04.063
Greif G, Greifová M, Karovičová J (2006) Effects of NaCl concentration and initial pH value on biogenic amine formation dynamics by Enterobacter spp. bacteria in model conditions. J Food Nutr Res 45:21–29
Greif G, Greifová M, Karovičová J, Kohajdová Z, Tomaška M (2008) Využitie IEX-HPLC na stanovenie metabolitov produkovaných baktériami mliečnej fermentácie. In: 17th International Conference “Analytical and Human Health”. Nový Smokovec, October 20–23. (CD ROM) (in Slovak)
Hernandez-Mendoza A, González-Córdova AF, Vallejo-Cordoba B, Garcia HS (2011) Effect of oral supplementation of Lactobacillus reuteri in reduction of intestinal absorption of aflatoxin B1 in rats. J Basic Microbiol 51:263–268. doi:10.1002/jobm.201000119
Ianniello RG, Zheng J, Zotta T, Ricciardi A, Gänzle MG (2015) Biochemical analysis of respiratory metabolism in the heterofermentative Lactobacillus spicheri and Lactobacillus reuteri. J Appl Microbiol 119:763–775. doi:10.1111/jam.12853
Kandler O (1983) Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 49:209–224
Kazatelová M (2003) Produkty metabolismu bakterií mléčného kvašení s antimikrobiální aktivitou. In: Bulletin potravinárskeho výskumu (Bulletin of Food Research) 42:67–73 (in Slovak) http://www.vup.sk/index.php?mainID=2&navID=36&version=1&volume=42&article=68
Kiňová Sepová H, Bilková A (2013) Isolation and identification of new lactobacilli from goatling stomach and investigation of reuterin production in Lactobacillus reuteri strains. Folia Microbiol 58:33–38. doi:10.1007/s12223-012-0166-x
Kohajdová Z, Karovičová J, Greif G (2008) Biogenic amines in food. Potravinárstvo 2:30–49 (in Slovak) http://www.potravinarstvo.com/dokumenty/potravinarstvo_no1_2008.pdf
Krauter H, Willke T, Vorlop KD (2012) Production of high amounts of 3-hydroxypropionaldehyde from glycerol by Lactobacillus reuteri with strongly increased biocatalyst lifetime and productivity. New Biotechnol 29:211–217. doi:10.1016/j.nbt.2011.06.015
Lavermicocca P, Valerio F, Visconti A (2003) Antifungal activity of phenyllactic acid aginst molds isolated from bakery products. Appl Environ Microbiol 69:634–664. doi:10.1128/AEM.69.1 634–640.2003
León Peláez AM, Serna Cataño CA, Quintero Yepes EA, Gamba Villarroel RR, De Antoni GL, Giannuzzi L (2012) Inhibitory activity of lactic and acetic acid on Aspergillus flavus growth for food preservation. Food Control 24:177–183. doi:10.1016/j.foodcont.2011.09.024
Li X, Bo J, Pan B (2007) Biotransformation of phenylpyruvic acid to phenyllactic acid by growing and resting cells of a Lactobacillus sp. Biotechnol Lett 29:593–597. doi:10.1007/s10529-006-9275-4
Linares DM, Del Río B, Ladero V, Martínez N, Fernández M, Martín MC, Alvarez MA (2012) Factor influencing biogenic amines accumulation in dairy products. Front Microbiol 180:1–10. doi:10.3389/fmicb.2012.00180
Lüthi-Peng Q, Dileme FB, Puhan Z (2002) Effect of glucose on glycerol bioconversion by Lactobacillus reuteri. Appl Microbiol Biotechnol 59:289–296. doi:10.1007/s00253-002-1002-z
Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J (2003) Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett 219:129–135. doi:10.1016/S0378-1097(02)01207-7
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation an tissue injury. Antioxid Redox Signal 20:1127–1166. doi:10.1089/ars.2012.5149
Molenaar D, Bosscher JS, ten Brink B, Driessen AJ, Konings WN (1993) Generation of a proton motive force by histidine decarboxylationn and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J Bacteriol 175:2864–2870
Morita H, Toh H, Fukuda S, Horikawa H, Oshima K, Suzuki T, Murakami M, Hisamatsu S, Kato Y, Takizawa T, Fukuokaa H, Yoshijmura T, Itoh K, O’ Sullivan DJ, McKay J, Ohno H, Kikuchi J, Masaoka T, Hattori M (2008) Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res 15:151–161. doi:10.1093/dnares/dsn009
Mu W, Yu S, Zhu L, Zhang T, Jiang B (2012) Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl Microbiol Biotechnol 95:1155–1163. doi:10.1007/s00253-012-4269-8
Pascual LM, Daniele MB, Pájaro C, Barberis L (2006) Lactobacillus species isolated from the vagina: identification, hydrogen peroxide production and nonoxynol-9 resistance. Contraception 73:78–81. doi:10.1016/j.contraception.2005.06.066
Renes E, Diezhandino I, Fernández D, Ferrazza RE, Tornadijo ME, Fresno JM (2014) Effect of autochthonous starter cultures on the biogenic amine content of ewe’s milk cheese throughout ripening. Food Microbiol 44:271–277. doi:10.1016/j.fm.2014.06.001
Rezaiki L, Cesselin B, Yamamoto Y, Vido K, van West E, Gaudu P, Gruss A (2004) Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol Microbiol 53:1331–1342. doi:10.1111/j.1365-2958.2004.04217.x
Schaefer L, Auchtung TA, Hermans KE, Whitehead D, Borhan B, Britton RA (2010) The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 156:1589–1599. doi:10.1099/mic.0.035642-0
Schnürer J, Magnusson J (2005) Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Tech 16:70–78. doi:10.1016/j.tifs.2004.02.014
Silla-Santos HSM (1996) Biogenic amines: their importance in food. Food Microbiol 29:213–231. doi:10.1016/0168-1605(95)00032-1
Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J (2008) Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14:166–171. doi:10.1016/j.anaerobe.2008.02.001
Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, Balderas MA, Saulnier DM, Mistretta TA, Devaraj S, Walter J, Versalovic J, Highlander SK (2014) From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol 6(7):1772–1789. doi:10.1093/gbe/evu137
Svanström Å, Boveri S, Boström E, Melin P (2013) The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Research Notes 6:1–9. doi:10.1186/1756-0500-6-464
Talarico TL, Dobrogosz WJ (1990) Purification and characterisation of glycerol dehydratasse from Lactobacillus reuteri. Appl Environ Microbiol 56:1195–1197
Thomas CM, Hong T, Van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J (2012) Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7:e31591. doi:10.1371/journal.pone.0031951
Tripathi T, Shahid M, Sobia F, Singh A, Khan HM, Khan RA, Siddiqui M (2010) Immune regulation by various facets of histamine in immunomodulation and allergic disorders. In: Khardori N, Khan RA, Tripathi T (eds) Biomedical aspect of histamine, 1st edn. Springer Science+Business Media B.V., Dordrecht, pp 133–147
Turpin W, Humbolt C, Thomas M, Guvot JP (2010) Lactobacilli as multifaceted probiotics with poorly disclosed molecular mechanisms: review. Int J Food Microbiol 143:87–102. doi:10.1016/j.ijfoodmicro.2010.07.032
Villegas E, Gilliland SE (1998) Hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis I at 5°C. J Food Sci 63:1070–1704
Wells JM (2011) Immunomodulatory mechanisms of lactobacilli. Microb Cell Factories 10(Suppl 1):S17. doi:10.1186/1475-2859-10-S1-S17
Xu HY, Tian WH, Wan CX, Jia LJ, Wang LY, Yuang J, Liu CM, Zeng M, Wei H (2008) Antagonistic potential against pathogenic microorganisms and hydrogen peroxide production of indigenous Lactobacilli isolated from vagina of Chinese pregnant women. Biomed Environ Sci 21:365–371. doi:10.1016/S0895-3988(08)60056-2
Zwietering MH, Jongenburger I, Van’t Reit K (1990) Modelling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Acknowledgements
This work was supported by a grant from the Ministry of Education of the Slovak Republic, VEGA 1/0569/16, by Comenius University grant UK/178/2015, and by Grant of Faculty of Pharmacy FAF/7/2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greifová, G., Májeková, H., Greif, G. et al. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri . Folia Microbiol 62, 515–524 (2017). https://doi.org/10.1007/s12223-017-0524-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0524-9