Abstract
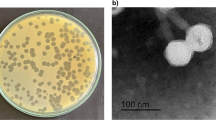
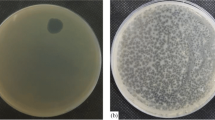
Pseudomonas aeruginosa is an opportunistic pathogen that causes serious infections, especially in patients with immunodeficiency. It exhibits multiple mechanisms of resistance, including efflux pumps, antibiotic modifying enzymes and limited membrane permeability. The primary reason for the development of novel therapeutics for P. aeruginosa infections is the declining efficacy of conventional antibiotic therapy. These clinical problems caused a revitalization of interest in bacteriophages, which are highly specific and have very effective antibacterial activity as well as several other advantages over traditional antimicrobial agents. Above all, so far, no serious or irreversible side effects of phage therapy have been described. Five newly purified P. aeruginosa phages named vB_PaeM_WP1, vB_PaeM_WP2, vB_PaeM_WP3, vB_PaeM_WP4 and vB_PaeP_WP5 have been characterized as potential candidates for use in phage therapy. They are representatives of the Myoviridae and Podoviridae families. Their host range, genome size, structural proteins and stability in various physical and chemical conditions were tested. The results of these preliminary investigations indicate that the newly isolated bacteriophages may be considered for use in phagotherapy.




Similar content being viewed by others
References
Ahiwale S, Tamboli N, Thorat K, Kulkarni R, Ackermann H, Kapadnis B (2011) In Vitro management of hospital Pseudomonas aeruginosa biofilm using indigenous T7-Like lytic phage. Curr Microbiol 62:335–340. doi:10.1007/s00284-010-9710-6
Ahiwale S, Prakash D, Gajbhiye M (2012) BVPaP-3, a T7-like lytic phage of Pseudomonas aeruginosa: it isolation and characterisation. Curr Microbiol 64:305–311
Balasubramanian D, Schneper L, Kumari H, Mathee K (2013) A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res 41:11–20. doi:10.1093/nar/gks1039
Clokie MRJ, Kropinski AM (2009) Bacteriophages: methods and protocols, molecular and applied aspects vol 1 and 2. Humana Press, Totowa, NJ
Coggan KA, Wolfgang MC (2012) Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr Issues Mol Biol 14:47–70
Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L (2010) Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis 201:1096–1104
Drulis-Kawa Z, Mackiewicz P, Kęsik-Szeloch A, Maciaszczyk-Dziubinska E, Weber-Dąbrowska B, Dorotkiewicz-Jach A, Augustyniak D, Majkowska-Skrobek G, Bocer T, Empel J, Kropinski AM (2011) Isolation and characterisation of KP34—a novel 8KMV-like bacteriophage for Klebsiella pneumoniae. Appl Microbiol Biotechnol 90:1333–1345. doi:10.1007/s00253-011-3149-y
Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM (2010) Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother 54(1):397–404
Fukuda K, Ishida W, Uchiyama J, Rachel M, Kato S, Morita T, Muraoka A, Sumi T, Matsuzaki S, Daibata M, Fukushima A (2012) Pseudomonas aeruginosa keratitis in mice: effects of topical bacteriophage KPP12 administration. PLoS ONE 7(10):e47742. doi:10.1371/journal.pone.0047742
Garbe J, Bunk B, Rohde M, Schobert M (2011) Sequencing and characterization of Pseudomonas aeruginosa phage JG004. BMC Microbiol 11:102
Hancock REW, Speert DP (2000) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat 3:247–255. doi:10.1054/drup.2000.0152
Hatfull GF, Hendrix RW (2011) Bacteriophages and their genomes. Curr Opin Virol 1(4):298–303. doi:10.1016/j.coviro.2011.06.009
Hirsch EB, Tam VH (2010) Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res 10:441–451
Hurley MN, Cámara M, Smith AR (2012) Novel approaches to the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. Eur Respiro J 40:1014–1023
Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 46–65. doi: 10.1128/MMBR.05007-11
Jończyk E, Kłak M, Międzybrodzki R, Górski A (2011) The influence of external factors on bacteriophages—review. Folia Microbiol 56:191–200. doi:10.1007/s12223-011-0039-8
Karumidze N, Thomas JA, Kvatadze N, Goderdzishvili M, Hakala KW, Weintraub ST, Alavidze Z, Hardies SC (2012) Characterization of lytic Pseudomonas aeruginosa bacteriophages via biological properties and genomic sequences. Appl Microbiol Biotechnol 94:1609–1617
Kęsik-Szeloch A, Drulis-Kawa Z, Weber-Dąbrowska B, Kassner J, Majkowska-Skrobek G, Augustyniak D, Łusiak-Szelachowska M, Żaczek M, Górski A, Kropinski AM (2013) Characterising the biology of novel lytic bacteriophages infecting multidrug resistant Klebsiella pneumoniae. Virol J 10:100
Krylov V, Shaburova O, Krylov S, Pleteneva E (2013) A genetic approach to the development of new therapeutic phages to fight Pseudomonas aeruginosa in wound infections. Viruses 5:15–53. doi:10.3390/v5010015
Lavigne R, Burkal’tseva MV, Robben J, Sykilinda NN, Kurochkina LP, Grymonprez B, Jonckx B, Krylov VN, Mesyanzhinov VV, Volckaerta G (2003) The genome of bacteriophage φKMV, a T7-like virus infecting Pseudomonas aeruginosa. Virology 312:49–59
Lecoutère E, Ceyssens PJ, Miroshnikov KA, Mesyanzhinov VV, Krylov VN, Noben JP, Robben J, Hertveldt K, Volckaert G, Lavigne R (2009) Identification and comparative analysis of the structural proteomes of φKZ and EL, two giant Pseudomonas aeruginosa bacteriophages. Proteomics 9:3215–3219. doi:10.1002/pmic.200800727
Liao KS, Lehman SM, Tweardy DJ, Donlan RM, Trautner BW (2012) Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J Appl Microbiol. doi:10.1111/j.1365-2672.2012.05432.x
McVay CS, Velásquez M, Fralick JA (2007) Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother 51:1934–1938
Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Van Bambeke F (2007) Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 13:560–578
Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L (2011) Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS ONE 6(2):e16963. doi:10.1371/journal.pone.0016963
Nakayama K, Kanaya S, Ohnishi M, Terawaki Y, Hayashi T (1999) The complete nucleotide sequence of φCTX, a cytotoxin-converting phage of Pseudomonas aeruginosa: implications for phage evolution and horizontal gene transfer via bacteriophages. Mol Microbiol 31(2):399–419
Pires D, Sillankorva S, Faustino A, Azeredo J (2011) Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res Microbiol. doi:10.1016/j.resmic.2011.06.010
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Tokajian S, Timani R, Issa N, Araj G (2012) Molecular characterization, multiple drug resistance, and virulence determinants of Pseudomonas aeruginosa isolated from Lebanon. Br Microbiol Res J 2(4):243–250
Veesenmeyer JL, Hauser AR, Lisboa T, Rello J (2009) Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 37(5):1777–1786. doi:10.1097/CCM.0b013e31819ff137
Wright A, Hawkins CH, Anggard EE, Harper DR (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357
Acknowledgments
The electron microscopy studies were performed in the Laboratory of Electron Microscopy, University of Warmia and Mazury in Olsztyn, Poland (electron microscope JEM 1400, installed as part of a project sponsored by EU Structural Funds).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwiatek, M., Mizak, L., Parasion, S. et al. Characterization of five newly isolated bacteriophages active against Pseudomonas aeruginosa clinical strains. Folia Microbiol 60, 7–14 (2015). https://doi.org/10.1007/s12223-014-0333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-014-0333-3