Abstract
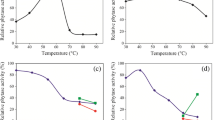
The presence of phytase activity was demonstrated in 26 strains of rabbit cecal bacteria. In 25 strains a low phytase activity, 0.10–0.62 μmol phosphate released per min per mg protein, was found. High activity (2.61 μmol/min per mg protein) was found in the strain PP2 identified as Enterococcus hirae. Phytase activity was cell-associated, being higher in the cell extract than in the cell walls. Extracellular phytase activity and cell-associated phosphatase activity were not detected. Phytase activity was optimal around pH 5.0, which is below the physiological cecal pH range. The K m determined using the Lineweaver-Burk plot was 0.19 μmol/mL. Cations Fe3+, Cu2+ and Zn2+ at 0.5 mmol/L decreased phytase activity in sonicated cells of E. hirae by 99.4, 90.7 and 96.5 %, respectively. In contrast, Mg2+ increased activity by 11.0 %. Characteristics of E. hirae phytase (pH optimum, K m, cation sensitivity) were similar to those of other bacterial phytases reported in the literature. Other bacteria with a high phytase activity may be present in the rabbit cecum but remain to be identified.
Similar content being viewed by others
References
Abriouel F., Lucas R., Ben Omar N., Valdivia E., Maqueda M., Martinez-Canamero M., Galvez A.: Enterocin AS-48RJ: a variant of enterocin AS-48 chromosomally encoded by Enterococcus faecium RJ16 isolated from food. Syst.Appl.Microbiol.28, 383–397 (2005).
Carabaño R., Piquer J.: The digestive system of the rabbit, pp. 1–16 in C. de Blas, J. Wiseman (Eds): The Nutrition of the Rabbit. CABI Publishing, Wallingford (UK) 1998.
Clark B., Holms W.H.: Control of the sequential utilization of glucose and fructose by Escherichia coli. J.Gen.Microbiol.95, 191–201 (1976).
D’silva C.G., Bae H.D., Yanke L.J., Cheng K.-J., Selinger L.B.: Localization of phytase in Selenomonas ruminantium and Mitsuokella multiacidus by transmission electron microscopy. Can.J.Microbiol.46, 391–395 (2000).
Dvořáková J.: Phytase: sources, preparation and exploitation. Folia Microbiol.43, 323–338 (1998).
Greiner R., Konietzny U., Jany K.D.: Purification and characterization of two phytases from Escherichia coli. Arch.Biochem.Biophys.303, 107–113 (1993).
Gulati H.K., Chadha B.S., Saini H.S.: Production of feed enzymes (phytase and plant cell wall hydrolyzing enzymes) by Mucor indicus MTCC 6333: purification and characterization of phytase. Folia Microbiol.52, 491–497 (2007).
Herbert D., Phipps P.J., Strange R.E.: Chemical analysis of microbial cells, pp. 209–344 in J.R. Norris, D.W. Ribbons (Eds): Methods in Microbiology, Vol.5B. Academic Press, London-New York 1971.
Igbasan F.A., Männer K., Miksch G., Borriss R., Farouk A., Simon O.: Comparative studies on the in vitro properties of phytases from various microbial origins. Arch.Anim.Nutr.53, 353–373 (2000).
Kerovuo J., Lauraeus M., Nurminen P., Kalkkinen N., Apajalahti J.: Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl.Environ.Microbiol.64, 2079–2085 (1998).
Maidak B.L., Larsen N., McCaughey M.J., Overbeek R., Olsen G.J., Fogel K., Blandy J., Woese C.R.: The ribosomal database project. Nucl.Acids Res.22, 3485–3487 (1994).
Marounek M., Petr O., Šimůnek J.: Monensin has no effect on growth and metabolism of Megasphaera elsdenii. Folia Microbiol.38, 383–386 (1993).
Marounek M., Dušková D., Skřivanová V.: Hydrolysis of phytic acid and its availability in rabbits. Brit.J.Nutr.89, 287–294 (2003).
Mateos G.G., de Blas C.: Minerals, vitamins and additives, pp. 145–175 in C. de Blas, J. Wiseman (Eds): The Nutrition of the Rabbit. CABI Publishing, Wallingford (UK) 1998.
Pandey A., Szakacs G., Soccol C.R., Rodriguez-Leon J.A., Soccol V.T.: Production, purification and properties of microbial phytases. Biores.Technol.77, 203–214 (2001).
Pavlova K., Gargova S., Hristozova T., Tankova Z.: Phytase from Antarctic yeast strain Cryptococcus laurentii AL27. Folia Microbiol.53, 29–34 (2008).
Peterson G.L.: A simplified method for analysis of inorganic phosphate in the presence of interfering substances. Anal.Biochem.84, 164–172 (1978).
Reddy N.R., Sathe S.K., Salunkhe D.K.: Phytates in legumes and cereals. Adv.Food Res.28, 1–92 (1982).
Scheuermann S.E., Lantzsch H.-J., Menke K.H.: In vitro und in vivo Untersuchungen zur Hydrolyse von Phytat. 1. Löslichkeit von Phytat. J.Anim.Physiol.Anim.Nutr.60, 55–63 (1988).
Shedova E., Lipasova V., Velikodvorskaya G., Ovadis M., Chernin L., Khmel I.: Phytase activity and its regulation in a rhizospheric strain of Serratia plymuthica. Folia Microbiol.53, 110–114 (2008).
Sirotek K., Marounek M., Rada V., Benda V.: Isolation and characterization of rabbit caecal pectinolytic bacteria. Folia Microbiol.46, 79–82 (2001).
Sirotek K., Santos E., Benda V., Marounek M.: Isolation, identification and characterization of rabbit caecal mucinolytic bacteria. Acta Vet. Brno72, 365–370 (2003).
Sirotek K., Rada V., Benda V., Marounek M.: Isolation and characterization of rabbit caecal xylanolytic bacteria. J Agrobiol.Ecol.1, 123–130 (2004).
Sirotek K., Marounek M., Suchorská O.: Activity and cellular localization of amylases of rabbit caecal bacteria. Folia Microbiol.51, 309–312 (2006).
Steer T.E., Gee J.N., Johnson I.T., Gibson G.R.: Biodiversity of human fecal bacteria isolated from phytic acid enriched chemostat fermenters. Curr.Iss.Intest.Microbiol.5, 23–30 (2004).
Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J.: 16S Ribosomal DNA amplification for phylogenetic study. J.Bacteriol.173, 697–703 (1991).
Yanke L.J., Bae H.D., Selinger L.B., Cheng K.-J.: Phytase activity of anaerobic ruminal bacteria. Microbiology144, 1565–1573 (1998).
Yanke L.J., Selinger L.B., Cheng K.-J.: Phytase activity of Selenomonas ruminantium: a preliminary characterization. Lett.Appl. Microbiol.29, 20–25 (1999).
Zamudio M., Gonzáles A., Medina J.A.: Lactobacillus plantarum phytase activity is due to non-specific acid phosphatase. Lett.Appl. Microbiol.32, 181–184 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marounek, M., Břeňová, N., Suchorská, O. et al. Phytase activity in rabbit cecal bacteria. Folia Microbiol 54, 111–114 (2009). https://doi.org/10.1007/s12223-009-0016-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-009-0016-7