Abstract
Increased nutrients have led cyanobacteria to become dominant in many ponds, lakes and reservoirs in many countries of the world. The occurrence and abundance of cyanobacterial population were monitored in a lake (known as Ishakha Lake) at Bangladesh Agricultural University campus, Mymensingh, Bangladesh. The hydrographic parameters such as water temperature, pH, chlorophyll a and nutrients (NO3–N and PO4–P) were recorded to find out their relationship with the cyanobacterial bloom formation. During the study period five species of cyanobacteria namely, Microcystis aeruginosa Kütz., M. wesenbergii Kom., M. botrys Teli., M. viridis (A. Br.) Lemm. and Anabaena circinalis Rabenh., were identified and among them M. aeruginosa was the dominant species during the bloom period. At the peak period of bloom, the highest cell density of M. aeruginosa was 1550 × 103 cells ml−1 which comprised 97.45 % among the blue-green algae and 96.84 % to the total phytoplankton. The initiation and persistence of natural bloom of cyanobacteria, especially Microcystis spp. was found to be controlled by relatively high temperature (>25.00 °C) and nutrients, especially high NO3–N (3.80 mg l−1) concentration. Temperature and NO3–N showed positive correlation with cyanobacterial cells abundance which were r = 0.62 and r = 0.92. Therefore, it could be said that temperature and NO3–N made a favorable circumstance to form cyanobacterial bloom in as Ishakha Lake. The Enzyme-linked Immunosorbent Assay revealed that the concentration of MCs 37,460.00 pg ml−1 at the peak period of bloom.
Similar content being viewed by others
1 Introduction
The microscopic planktonic algae play an important role in the environment as critical food for fish and crustaceans. However, in some situations algal blooms can have a negative effect causing economic losses to aquaculture and fisheries (Hallegraeff 1993). Recently, the geographic distributions as well as the intensity of these blooms have increased (Hallegraeff et al. 1988; Han et al. 1992). Cyanobacteria are often reported to be toxic in fresh, brackish and marine waters in many parts of the world (WHO 1984). Blooms have known to occur in waters where the nutrient levels are elevated and often associated with the use of fertilizers. The most bloom forming cyanobacteria are Microcystis, Anabaena, Nodularia, Planktothrix and Aphanizomenon. Such blooms have caused numerous animal poisoning and mortality of wildlife and domestic animals (Carmichael 1992). Fatalities and severe illness resulting from cyanobacterial bloom have been reported in many countries (Carmichael and Falconer 1993).
Excessive phytoplankton growth is a serious problem for aquaculture, because it is known to negatively affect water quality in fish ponds in at least three ways (Smith 1985). First, it can lead to chronic oxygen deficits. Second, dense algal blooms collapse periodically leading to decomposition of the dead algae resulting in fish kills due to anoxia (Boyd et al. 1975; Barica 1975) as well as due to high levels of ammonia (Seymour 1980; Tucker et al. 1984). Third, blue-green algae exude chemicals- geosmin and methyl-isoborneol that taint fish flesh, i.e., causing off-flavor of fish (Brown and Boyd 1982; Armstrong et al. 1986).
In Bangladesh, with gradual intensification of aquaculture (with the use of fish-feeds and fertilizers) fish farmers are experiencing many harmful and noxious algal blooms. Furthermore, incidents of hepatitis, liver cancer, asthma, skin rashes and other allergic problems are increasing unexpectedly fast in this densely populated developing country both in rural and urban areas. There are some reports that cyanotoxins have direct or indirect relationship with some of the above diseases (Yu 1989; Turner et al. 1990; Bell and Codd 1994; Ueno et al. 1996). Cyanobacteria forms blooms round the year with the peak abundance in spring and summer in many ponds, lakes and other reservoirs of the country and this bloom might have some relationship with various water quality parameters.
Growth of phytoplankton is one of the most important factors determining the abundance and distribution of microalgae (Admiraal 1977). Growth of phytoplankton in waterbodies is controlled by various environmental factors, such as temperature, pH, light, nutrients, stratification, water turbidity, etc. (Tomas 1978; Uye and Takamatsu 1990). Nielson and Tonseth (1991) suggested that temperature was an important limiting factor in the initiation of blooms of Gyrodinium aureolum Hulburt in north European waters. Nutrients are one of the most important environmental factors that influence algal growth (Okaichi et al. 1989). Ono (1988) found that a nitrogen concentration of 0.3 mg l−1 and a phosphorus concentration of 0.03–3.0 mg l−1 were optimal for the growth of Fibrocapsa japonica Toriumi and Takano. Ecological and physiological parameters of phytoplankton have been suggested to vary from species to species as well as from strain to strain. Honjo (1993) reported five different optimum temperatures for five different strains of Heterosigma akashiwo (Hada). The autecological knowledge of cyanobacteria would be useful in predicting possible danger periods, taking precautions and determining the feasibility of control measures. However, this study was conducted to find out the effect of physic-chemical factors on the bloom formation of cyanobacteria and qualitative and quantitative contribution of different species of cyanobacteria to the bloom in Ishakh Lake.
2 Materials and methods
2.1 Study area and sampling
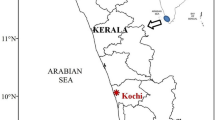
The present study was conducted in a lake at Bangladesh Agricultural University campus, commonly known as Ishakha Lake, Mymensingh, Bangladesh (Fig. 1). Lake water is used for fish culture, bathing and washing clothes. Every year it experiences periodic cyanobacterial bloom. The lake receives kitchen wastes and decomposed organic nutrients through drains of three residential halls of university students situated 20 m from the inlet. Samples were collected directly in one liter plastic bottles from five different randomly selected positions of the pond. Bi-weekly sampling started at the 1st January 2001 and continued up to the initiation of bloom and then daily during the bloom period (22 February–4 March 2001). Just after collection, the samples were preserved in 5 % buffered formalin.
2.2 Analysis of water quality parameters
Surface water temperature and pH were determined using a celsius thermometer and an electronic pH meter (Jenway 3020, Germany), respectively. Nitrate–nitrogen (NO3–N) and phosphate–phosphorus (PO4–P) were measured using a HACH kit (DR/2010, a direct reading spectrophotometer) using high range chemicals (NitraVer 5 Nitrate Reagent Powder Pillows for NO3–N, and PhosVer 3 Phosphate Reagent Powder Pillows for PO4–P analysis). Chlorophyll a was determined spectrophotometrically (Milton Roy Spectronic, 1001, Germany) after acetone extraction (APHA 1992). During peak bloom a phytoplankton sample was analyzed for testing microcystins (MCs) concentration following Enzyme-linked Immunosorbent Assay.
2.3 Enzyme-linked Immunosorbent Assay
From each sample, 10 ml water was poured in a 12 ml polyvinyl vial and 1/100 volume of 10 % sodium azide was added and frozen in a deep freezer. The samples were freeze–thawed twice, and then filtered through glass fiber filters (Whatman GF/C, 25 mm in diameter) and used for enzyme-linked immunosorbent assay (ELISA). The water samples or microcystins LR (MC-LR) standard were mixed with an appropriate dilution of the anti-MC-LR MAb M8H5, and then added to a 96-well microtiter plate coated with MC-LR-bovine serum albumin conjugate. After washing, the bound MAb was detected with horseradish peroxidase-labeled goat anti-mouse IgG (TAGO 4550) plus substrate (0.1 mg ml−1 3,3′-5,5′-tetramethyl benzidine, 0.005 % H2O2 in 0.1 M acetate buffer pH 5), resulting in an absorption measurement at 450 nm. The concentrations of MCs used when examining the ELISA data were an average of two triplicate estimations expressed as picogram per milliliter (pg ml−1) by MC-LR.
2.4 Phytoplankton study
For species identifications, buffered formalin preserved sample was gently shaken to resuspend all materials and was allowed to settle for 1 min. Then 2–3 drops were removed from the middle of the sample and placed on a glass slide. Taxonomic determination of cyanobacteria was performed with a phase-contrast microscope (Olympus, Japan) at 100–400 X, with brightfield and phase contrast illumination on living materials and on samples preserved with formaldehyde. Identification was done following the morphological characteristics described by Komarek (1958), Skulberg and Skulberg (1985). The quantitative estimation of phytoplankton was done by Sedgewick–Rafter counting chamber (S–R cell) following the method described by Stirling (1985).
3 Results
The temperature increased from 18.50 to 32.50 °C during the study period. The cyanobacterial bloom peaked during high temperature (>29 °C). The average temperature was (28.00–31.50 °C) during peak bloom period (26 February–1 March). As the temperature continued to increase from 28.60 to 32.50 °C cyanobacteria seemed to decrease rapidly. The pH fluctuation was slight ranging from 7.50 to 10.00 (Fig. 2). Higher cell abundance of cyanobacteria was found when the pH was approximately 8.80. Chlorophyll a paralleled the cyanobacterial cell density. The lowest value of chlorophyll a (0.60 mg l−1) was recorded at the beginning of this study. During the bloom period the range of chlorophyll a fluctuated from 2.30 to 27.65 mg l−1 (Fig. 2). Nitrate (NO3–N) concentration ranged from 1.10 to 3.80 mg l−1, and the higher (2.2–3.8 mg l−1) concentration was observed during the bloom period. Fluctuation of phosphate (PO4–P) concentration was small ranging from 0.30 to 1.00 mg l−1 (Fig. 3).
The cyanobacterial cell abundance was low (15.80 × 103–34.20 × 103 cells ml−1) during the 1st sample periods (1 January–15 January). Cell abundance started to increase gradually at the middle of February and cyanobacterial bloom was starting to be visible with naked eyes from 22 February which continued until the first week of March (4 March, 2001). During the initiation of the bloom, the cyanobacterial cell density was 15 times higher (226.45 × 103 cells ml−1) than the base population (population prior to February 22) (Table 1). The abundance began to increase rapidly from February 22 and peaked within 4 days forming green paint like scum on the surface of the water body. The cell abundance was about seven times higher (1590.55 × 103 cells ml−1) than the cell abundance of early bloom. This abundance then began to decline after 28 of February reaching lowest level (340.00 × 103 cells ml−1) on March 4.
During the peak bloom cyanobacteria contributed 99.37 % of the total phytoplankton population (Table 1). The cell density of other phytoplankton groups, like Chlorophyceae, Bacillariophyceae and Euglenophyceae decreased with increasing cyanobacterial cell density. The cell density of Bacillariophyceae and Euglenophyceae were absent with very low density of Chlorophyceae when the cell density of cyanobacteria reached 1006.95 × 103 cells ml−1.
Five species of cyanobacteria Microcystis aeruginosa, M. wesenbergii, M. botrys, M. viridis and Anabaena circinalis were identified and among them M. aeruginosa was dominant during the bloom period (22 February to 4 March). At the peak of bloom period, the highest cell abundance of M. aeruginosa was 1550.00 × 103 cells ml−1 which comprised 97.45 % among the cyanobacteria (Table 2). During the bloom period, M. aeruginosa cell abundance was found to be began increasing when the cell density of M. wesenbergii began to decrease.
During the peak bloom planktivorous fish silver carp (Hypophthalmichthys molitrix) was found to be died the morning. The Enzyme-linked Immunosorbent Assay revealed that the concentration of MCs 37,460.00 pg ml−1 at the peak period of bloom when M. aeruginosa was 1550.00 × 103 cells ml−1 which comprised 96.84 % of the total phytoplankton.
4 Discussion
The Ishakha Lake, in which this study was carried out, is typical of many other shallow, warmer and eutrophic ponds or lakes in Bangladesh that regularly produce dense bloom of planktonic cyanobacteria from spring to summer. By the last week of February 2001, a typical surface bloom of cyanobacteria started to be observed whose intensity was very high (Fig. 4a, b), then the bloom reached peak by the end of last week of February, and at that time the surface scum of cyanobacteria covered most part of the pond (Fig. 4c). At the beginning of March the bloom was found to be declined (Fig. 4d). The recorded cyanobacterial cell density during the peak bloom was 1590.55 × 103 cells ml−1 in the studied lake. Park et al. (1996) recorded the cell density of Microcystis 6.70 × 105 cells ml−1 during the bloom period in Lake Suwa, Japan which is about two times lower than the population which we found in our present study. M. aeruginosa was the dominant species representing 96.84 % of the total phytoplankton and 97.45 % among Cyanophyceae. This result is similar with the findings of Oudra et al. (1998) who recorded 95.00 % M. aeruginosa in a cyanobacterial bloom in eutrophic Lalla Takerkousta reservoir in Morocco.
The initiation and persistence of Microcystis natural bloom in the lake, seemed to be determined by relatively high temperatures (28.00–30.00 °C) in spring season, alkaline pH, high nutrients concentration, especially NO3–N concentration, and bright sunlight. In our present study, the effect of temperature on the bloom formation of M. aeruginosa agrees fairly well with Eloff (1980) who also found that temperature of 28.80–30.50 °C was optimal for the growth of M. aeruginosa. Watanabe and Oishi (1985) also found faster growth of M. aeruginosa at temperature 32.00 °C under culture condition. Alkaline water may have promoted the outbreak of Microcystis bloom. In the present study, the pH ranged from 8.20 to 8.80 during the bloom period. Van der Westhuizen and Eloff (1983) also found highest growth rate of M. aeruginosa in culture media with pH around 9.0 during batch culture.
Cyanobacteria cell abundance increased with the increase in NO3–N concentration and dropped with the decline in NO3–N. Generally, occasional rainwater during spring months and heavy rainfall in the monsoon months flushes organic materials from the adjacent fallow lands and from the household drains into the lake. Nutrients from decomposition of those organic materials trigger cyanobacterial bloom. During the study period rainfall occurred only once at the beginning of the spring and earlier than other years. These increased nutrients might have created favorable condition for the out-break of cyanobacteria, mainly M. aeruginosa blooms in this lake. The concentration of NO3–N and cyanobacterial cell abundance showed highly positive correlation (r = 0.92). Similar results were reported by Park et al. (1993) who suggested that increase of NO3–N concentration favored the growth of Microcystis at Lake Suwa in Japan. Furthermore, toxic Microcystis population collapsed when NO3–N concentration decreased (Utkilen et al. 1996). Microcystis may not require high concentration of PO4–P for their growth as in our study where Microcystis bloom occurred in relatively low PO4–P concentration. Gerloff et al. (1952) also found that Microcystis had a relatively low phosphorus requirement for its growth.
A negative relationship between M. aeruginosa and M. wesenbergii cell abundance was found which indicating M. aeruginosa might have some growth inhibitory substances and that substances may be suppressed the growth of M. wesenbergii. Similarly, Lam and Silvester (1979) also found negative correlation between Cyanophyceae and Chlorophyceae. Similar evidence was observed by Hossain (1989) who described that Anabaena showed more negative correlation with others; Nostoc and Aphanocapsa exhibited more negative correlation with some other genera and species of phytoplankton. However, during the peak bloom of M. aeruginosa, fish mortality occurred which might be related with the toxin produced by M. aeruginosa, or suffocation due low concentration of dissolved oxygen or by gill clogging by the high dense cyanobacteria of lake water, as it is reported elsewhere in the world.
5 Conclusion
It could be said that temperature and NO3–N are the most important environmental factors which make favorable condition for bloom formation of cyanobacterial in lake.
References
Admiraal W (1977) Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar Biol 39:1–9
APHA (American Public Health Association) (1992) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Armstrong MS, Boyd CE, Lovell RT (1986) Environmental factors affecting flavor of channel catfish from production ponds. Progress Fish Cult 48:113–119
Barica J (1975) Collapse of algal blooms in prairie pothole lakes: their mechanism and biological impact. Verhandlungen Internationaler Vereinigung fuer Theoretische und Angewandt e Limnologie 19:606–615
Bell SG, Codd GA (1994) Cyanobacterial toxins and human health. Rev Med Microbiol 5:256–264
Boyd CE, Prather EE, Parks RW (1975) Sudden mortality of a massive phytoplankton bloom. Weed Sci 23:61–67
Brown SW, Boyd CE (1982) Off-flavor in channel catfish from commercial ponds. Trans Am Fish Soc 111:379–383
Carmichael WW (1992) Cyanobacterial secondary metabolites—the cyanotoxins. J Appl Bacteriol 72:445–459
Carmichael WW, Falconer IR (1993) Disease related to freshwater algal toxins, and control measures. In: Falconer IR (ed) Algal toxins in sea food and drinking water. Academic Press, London, pp 187–209
Eloff JN (1980) Autecological studies on Microcystis. In: Carmichael WW (ed) The water environment, algal toxins and health. Plenum Press, New York, pp 71–96
Gerloff GC, Fitzgerald GP, Skoog F (1952) The mineral nutrition of Microcystis aeruginosa. Am J Bot 39:26–32
Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32:79–99
Hallegraeff GM, Steffensen DA, Wetherbee R (1988) Three estuarine Australian dinoflagellates that can produce paralytic shellfish toxins. J Plankton Res 10:533–541
Han MS, Jean JK, Kim YO (1992) Occurrence of dinoflagellate Alexandrium tamarense, a causative organism of paralytic shellfish poisoning in Chinhae Bay, Korea. J Plankton Res 14:1581–1592
Honjo T (1993) Overview on bloom dynamics and physiological ecology of Heterosigma akashiwo. In: Smayda TJ, Shimizu Y (eds) Toxic phytoplankton blooms in the sea. Elsevier, New York, pp 33–41
Hossain MA (1989) A study on the feeding ecology of Clarias batrachus (Linn), and Anabas testudineus (Bloch). Ph.D. Thesis. Department of Aquaculture and Management, BAU, Mymensingh, p 308
Komarek J (1958) Die taxonomische revision der planktischen blaualgen der Tschechoslawakei. In: Komarek J, Ettl H (eds) Algologische studien. Tschechoslawakishe Akademie der Wissenschaften, Prague, pp 10–206
Lam CWY, Silvester WB (1979) Growth interactions among blue-green (Anabaena oscillarioides, Microcystis aeruginosa) and green (Chlorella sp.) algae. Hydrobiologia 63:135–143
Nielson MV, Tonseth CP (1991) Temperature and salinity effect on growth and chemical composition of Gyrodinium aureolum Hulburt in culture. J Plankton Res 13:389–398
Okaichi T, Hiragi SJ, Hasui A (1989) The role of iron in the outbreaks of Chattonella red tide. In: Okaichi T (ed) Red tides. Elsevier, pp 353–356
Ono C (1988) Cell cycles and growth rates of red tide organisms in Harima-Nada area, eastern part of Seto Inland Sea, Japan. Bull Akashiwo Res Inst Kagawa Prefect 3:1–67
Oudra B, Mohammed L, Brahim S, Vitor V, Halim Z, Maria-El A, Jacqueline D (1998) Occurrence of hepatotoxic Microcystis aeruginosa waterblooms in a eutrophic Moroccan lake reservoir. In: Reguera B, Blanco J, Fernandez ML, Wyatt T (eds) Harmful algae. Xunta de Galicia and Intergovernmental Oceanographic Commission, UNESCO, pp 29–31
Park HD, Watanabe MF, Harada KI, Suzuki M, Hayashi H, Okino T (1993) Seasonal variations of Microcystis species and toxic heptapeptide microcystins in Lake Suwa. Environ Toxicol Water Qual 8:425–435
Park HD, Chie I, Mariyo FW, Ken-ichi H, Tokio O, Hidetake H (1996) Seasonal changes of toxic Microcystis and amount of microcystin in Lake Suwa, Japan. In: Yasumoto T, Oshima Y, Fukuyo Y (eds) Harmful and toxic algal blooms. Intergovernmental Oceanographic Commission, UNESCO, pp 555–558
Seymour EA (1980) The effect and control of algal blooms in fish ponds. Aquaculture 19:55–74
Skulberg OM, Skulberg R (1985) Planktic species of Oscillatoria (Cyanophyceae) from Norway—characterization and classification. Arch Hydrobiol 71:157–174
Smith DW (1985) Biological control of excessive phytoplankton growth and the enhancement of aquacultural production. Can J Fish Aquat Sci 42:1940–1945
Stirling HP (1985) Chemical and biological methods of water analysis for aquaculturists. Institute of Aquaculture, University of Stirling, Scotland
Tomas CR (1978) Olisthodiscus luteus (Chrysophyceae) I. Effects of salinity and temperature on growth, motility and survival. J Phycol 14:309–313
Tucker CS, Lloyd SW, Busch RL (1984) Relationship between phytoplankton periodicity and the concentration of total and unionized ammonia in channel catfish ponds. Hydrobiologia 111:75–79
Turner PC, Gammie AJ, Hollinrake K, Codd GA (1990) Pneumonia associated with contact with cyanobacteria. Br Med J 300:1440–1441
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, Chen GC, Yu SZ (1996) Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17:1317–1321
Utkilen H, Skulberg OM, Underdhal B, Gjolme N, Skulberg R, Kotai J (1996) The rise and fall of a toxigenic population of a Microcystis aeruginosa (Cyanophyceae/Cyanobacteria)—a decade of observations in Lake Akersvatnet, Norway. Phycologia 35:189–197
Uye S, Takamatsu K (1990) Feeding interactions between planktonic copepods and red-tide flagellates from Japanese coastal waters. Mar Ecol Prog Ser 59:97–107
Van der Westhuizen AJ, Eloff JN (1983) Effect of culture age and pH of culture medium on the growth and toxicity of the blue-green alga Microcystis aeruginosa. Z Pflanzenphysiol 110:157–163
Watanabe MF, Oishi S (1985) Effect of environmental factors on toxicity of cyanobacterium Microcystis aeruginosa under culture conditions. Appl Environ Microbiol 49:1342–1344
World Health Organization (1984) Aquatic (marine and freshwater) biotoxins. Environmental Health Criteria 37. WHO, Geneva
Yu SZ (1989) Drinking water and primary liver cancer. In: Tang Z, Wu YMC, Xia SS (eds) Primary liver cancer. China Academic Publishers, New York, pp 30–37
Acknowledgments
We would like to thank to the staff members at algal phytoplankton laboratory King Abdulaziz University (KAU) for helpful discussion to write this manuscript. This study was conducted as a part of the BAURES (Bangladesh Agricultural University Research System) funded research project on “Noxious and Toxic Phytoplankton in Ponds”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Affan, M.A., Touliabah, H.ES., Al-Harbi, S.M. et al. Influence of environmental parameters on toxic cyanobacterial bloom occurrence in a Lake of Bangladesh. Rend. Fis. Acc. Lincei 27, 473–481 (2016). https://doi.org/10.1007/s12210-016-0502-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-016-0502-1