Abstract
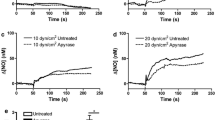
Endothelial dysfunction, characterized by decreased production or availability of nitric oxide (NO), is widely believed to be the hallmark of early-stage atherosclerosis. In addition, hypercholesterolemia is considered a major risk factor for development of atherosclerosis and is associated with impaired flow-induced dilation. However, the mechanism by which elevated cholesterol levels leads to decreased production of NO is unclear. NO is released in response to shear stress and agonist-evoked changes in intracellular calcium. Although calcium signaling is complex, we have previously shown that NO production by endothelial nitric oxide synthase (eNOS) is preferentially activated by calcium influx via store-operated channels. We hypothesized that cholesterol enrichment altered this signaling pathway (known as capacitive calcium entry; CCE) ultimately leading to decreased NO. Our results show that cholesterol enrichment abolished ATP-induced eNOS phosphorylation and attenuated the calcium response by the preferential inhibition of CCE. Furthermore, cholesterol enrichment also inhibited shear stress-induced NO production and eNOS phosporylation, consistent with our previous results showing a significant role for ATP autocrine stimulation and subsequent activation of CCE in the endothelial flow response.









Similar content being viewed by others
References
Ambudkar, I. S., B. C. Bandyopadhyay, X. B. Liu, T. P. Lockwich, B. Paria, and H. L. Ong. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium 40:495–504, 2006.
Andrews, A. M., D. Jaron, D. G. Buerk, and K. A. Barbee. Shear stress-induced NO production is dependent on ATP autocrine signaling and capacitative calcium entry. Cell Mol. Bioeng. 7:510–520, 2014.
Andrews, A. M., D. Jaron, D. G. Buerk, P. L. Kirby, and K. A. Barbee. Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide 23:335–342, 2010.
Bastiaanse, E. M. L., K. M. Hold, and A. VanderLaarse. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 33:272–283, 1997.
Bialecki, R. A., and T. N. Tulenko. Excess membrane cholesterol alters calcium channels in arterial smooth muscle. Am. J. Physiol. 257:C306–C314, 1989.
Boittin, F. X., F. Gribi, K. Serir, and J. L. Beny. Ca2+-independent PLA2 controls endothelial store-operated Ca2+ entry and vascular tone in intact aorta. Am. J. Physiol. Heart Circ. Physiol. 295:H2466–H2474, 2008.
Bowles, D. K., C. L. Heaps, J. R. Turk, K. K. Maddali, and E. M. Price. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J. Appl. Physiol. 96:2240–2248, 2004.
Buga, G. M., M. E. Gold, J. M. Fukuto, and L. J. Ignarro. Shear-stress induced release of nitricoxide from endothelial-cells grown on beads. Hypertension 17:187–193, 1991.
Cabral, P. D., N. J. Hong, and J. L. Garvin. ATP mediates flow-induced NO production in thick ascending limbs. Am. J. Physiol. Ren. Physiol. 303:F194–F200, 2012.
Casino, P. R., C. M. Kilcoyne, A. A. Quyyumi, J. M. Hoeg, and J. A. Panza. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation 88:2541–2547, 1993.
Chun, Y. S., S. Shin, Y. Kim, H. Cho, M. K. Park, T. W. Kim, S. V. Voronov, G. Di Paolo, B. C. Suh, and S. Chung. Cholesterol modulates ion channels via down-regulation of phosphatidylinositol 4,5-bisphosphate. J. Neurochem. 112:1286–1294, 2010.
Cohen, R. A., F. Plane, S. Najibi, I. Huk, T. Malinski, and C. J. Garland. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc. Natl. Acad. Sci. USA 94:4193–4198, 1997.
Dedkova, E. N., and L. A. Blatter. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J. Physiol. Lond. 539:77–91, 2002.
Fang, Y., R. M. Emile, E. Hsieh, H. Osman, S. M. Hashemi, P. F. Davies, G. H. Rothblat, R. L. Wilensky, and I. Levitan. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. CircRes 98:1064–1071, 2006.
Feron, O., C. Dessy, S. Moniotte, J. P. Desager, and J. L. Balligand. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 103:897–905, 1999.
Ferroni, P., S. Basili, V. Paoletti, and G. Davi. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr. Metab. Carbiovasc. Dis. 16:222–233, 2006.
Fisslthaler, B., S. Dimmeler, C. Hermann, R. Busse, and I. Fleming. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand 168:81–88, 2000.
Flavahan, N. A. Atherosclerosis or lipoprotein-induced endothelial dysfunction—potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation 85:1927–1938, 1992.
Giannattasio, C., A. A. Mangoni, M. Failla, S. Carugo, M. L. Stella, P. Stefanoni, G. Grassi, C. Vergani, and G. Mancia. Impaired radial artery compliance in normotensive subjects with familial hypercholesterolemia. Atherosclerosis 124:249–260, 1996.
Glagov, S., E. Weisenberg, C. K. Zarins, R. Stankunavicius, and G. J. Kolettis. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316:1371–1375, 1987.
Graziani, A., V. Bricko, M. Carmignani, W. F. Graier, and K. Groschner. Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc. Res. 64:234–242, 2004.
Hashimoto, M., K. Shinozuka, Y. Tanabe, H. M. Shahdat, S. Gamoh, Y. M. Kwon, Y. Tanaka, M. Kunitomo, and S. Masumura. Long-term supplementation with a high cholesterol diet decreases the release of ATP from the caudal artery in aged rats. Life Sci. 63:1879–1885, 1998.
Hayashi, T., M. Naito, T. Ishikawa, M. Kuzuya, C. Funaki, T. Tateishi, K. Asai, H. Hidaka, and F. Kuzuya. Beta-migrating very low density lipoprotein attenuates endothelium-dependent relaxation in rabbit atherosclerotic aortas. Blood Vessel. 26:290–299, 1989.
Hong, D., D. Jaron, D. G. Buerk, and K. A. Barbee. Heterogeneous response of microvascular endothelial cells to shear stress. Am. J. Physiol. Heart Circul. Physiol. 290:H2498–H2508, 2006.
Hong, D., D. Jaron, D. G. Buerk, and K. A. Barbee. Transport-dependent calcium signaling in spatially segregated cellular caveolar domains. Am. J. Physiol. Cell Physiol. 294:C856–C866, 2008.
Jan, C. R., C. M. Ho, S. N. Wu, and C. J. Tseng. Mechanism of rise and decay of thapsigargin-evoked calcium signals in MDCK cells. Life Sci. 64:259–267, 1999.
Jansen, M., V. M. Pietiarinen, H. Polonen, L. Rasilainen, M. Koivusalo, U. Ruotsalainen, E. Jokitalo, and E. Ikonen. Cholesterol substitution increases the structural heterogeneity of caveolae. J. Biol. Chem. 283:14610–14618, 2008.
Kuchan, M. J., H. Jo, and J. A. Frangos. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am. J. Physiol. 267:C753–C758, 1994.
Lee, A. K., V. Yeung-Yam-Wah, F. W. Tse, and A. Tse. Cholesterol elevation impairs glucose-stimulated Ca(2+) signaling in mouse pancreatic beta-cells. Endocrinology 152:3351–3361, 2011.
Levitan, I., A. E. Christian, T. N. Tulenko, and G. H. Rothblat. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 115:405–416, 2000.
Li, Y. K., M. T. Ge, L. Ciani, G. Kuriakose, E. J. Westover, M. Dura, D. F. Covey, J. H. Freed, F. R. Maxfield, J. Lytton, and I. Tabas. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids—Implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 279:37030–37039, 2004.
Lin, S., K. A. Fagan, K. X. Li, P. W. Shaul, D. M. F. Cooper, and D. M. Rodman. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J. Biol. Chem. 275:17979–17985, 2000.
Linder, A. E., L. P. McCluskey, K. R. Cole, 3rd, K. M. Lanning, and R. C. Webb. Dynamic association of nitric oxide downstream signaling molecules with endothelial caveolin-1 in rat aorta. J. Pharmacol. Exp. Ther. 314:9–15, 2005.
Lockwich, T. P., X. B. Liu, B. B. Singh, J. Jadlowiec, S. Weiland, and I. S. Ambudkar. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 275:11934–11942, 2000.
Mochizuki, S., M. Goto, Y. Chiba, Y. Ogasawara, and F. Kajiya. Flow dependence and time constant of the change in nitric oxide concentration measured in the vascular media. Med. Biol. Eng. Comput. 37:497–503, 1999.
Neishi, Y., S. Mochizuki, T. Miyasaka, T. Kawamoto, T. Kume, R. Sukmawan, M. Tsukiji, Y. Ogasawara, F. Kajiya, T. Akasaka, K. Yoshida, and M. Goto. Evaluation of bioavailability of nitric oxide in coronary circulation by direct measurement of plasma nitric oxide concentration. Proc. Natl. Acad. Sci. USA 102:11456–11461, 2005.
Pani, B., and B. B. Singh. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 45:625–633, 2009.
Peterson, T. E., V. Poppa, H. Ueba, A. Wu, C. Yan, and B. C. Berk. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. CircRes 85:29–37, 1999.
Plotnick, G. D., M. C. Corretti, R. A. Vogel, R. Hesslink, and J. A. Wise. Effect of supplemental phytonutrients on impairment of the flow-mediated brachial artery vasoactivity after a single high-fat meal. J. Am. Coll. Cardiol. 41:1744–1749, 2003.
Saini, H. K., A. S. Arneja, and N. S. Dhalla. Role of cholesterol in cardiovascular dysfunction. Can. J. Cardiol. 20:333–346, 2004.
Shaul, P. W. Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J. Physiol. 547:21–33, 2003.
Troup, G. M., Y. Xie, K. Boesze-Battaglia, Y. Huang, T. Kirk, F. Hanley, and T. N. Tulenko. Membrane Cholesterol and the Formation of Cholesterol Domains in the Pathogenesis of Cardiovascular Disease. V C H Verlag Gmbh: Wiley, p. 25, 2004.
Troup, G. M., Y. Xie, K. Boesze-Battaglia, Y. Huang, T. Kirk, F. Hanley, and T. N. Tulenko. Membrane cholesterol and the formation of cholesterol domains in the pathogenesis of cardiovascular disease. Macromol. Symp. 219:25–38, 2005.
Tulenko, T. N., M. Chen, P. E. Mason, and R. P. Mason. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid Res. 39:947–956, 1998.
Vogel, R. A., M. C. Corretti, and G. D. Plotnick. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 79:350–354, 1997.
Wang, T. K., Z. Chen, X. Wang, J. Y. J. Shyy, and Y. Zhu. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. BBA Mol. Cell Biol. Lipids 1761:1182–1190, 2006.
Xu, Y., R. H. Henning, J. J. van der Want, A. van Buiten, W. H. van Gilst, and H. Buikema. Disruption of endothelial caveolae is associated with impairment of both NO- as well as EDHF in acetylcholine-induced relaxation depending on their relative contribution in different vascular beds. Life Sci. 80:1678–1685, 2007.
Yuan, Y., L. K. Verna, N. P. Wang, H. L. Liao, K. S. Ma, Y. Wang, Y. Zhu, and M. B. Stemerman. Cholesterol enrichment upregulates intercellular adhesion molecule-1 in human vascular endothelial cells. BBA Mol. Cell Biol. Lipids 1534:139–148, 2001.
Zhang, Q., J. E. Church, D. Jagnandan, J. D. Catravas, W. C. Sessa, and D. Fulton. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb. Vasc. Biol. 26:1015–1021, 2006.
Zidovetzki, R., and I. Levitan. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta Biomembr. 1768:1311–1324, 2007.
Acknowledgments
We would like to acknowledge the following funding sources for this project: NIH/HL068164 (DJ, KAB), NSF/BES0301446 (DJ, KAB), NSF/CBET0730547 (DJ, KAB), NIH U01HL116256 (DJ, KAB, DGB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ms. Muzorewa, Ms. Zaccheo, and Dr. Buerk have nothing to disclose. Dr. Andrews, Dr. Jaron and Dr. Barbee have a patent 8,828,711 issued.
Ethical standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Additional information
Associate Editor William H. Guilford oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Andrews, A.M., Muzorewa, T.T., Zaccheo, K.A. et al. Cholesterol Enrichment Impairs Capacitative Calcium Entry, eNOS Phosphorylation & Shear Stress-Induced NO Production. Cel. Mol. Bioeng. 10, 30–40 (2017). https://doi.org/10.1007/s12195-016-0456-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-016-0456-5