Abstract
The peptidyl transferase antibiotic sparsomycin can induce efficient ribosomal translocation in the absence of EF-G and GTP. However, how the antibiotic facilitates the translocation is unclear. Here, two models of antibiotic-induced translocation are considered, in which the interaction of the antibiotic with the peptidyl-tRNA in the A/P state, besides behaving as a pawl in the “Brownian ratchet”, also has an effect of compromising the interaction of the 30S subunit with the mRNA-tRNA complex (Model I) or facilitating the forward 30S head rotation which results in the ribosomal unlocking (Model II). It is shown that although the results obtained with Model I can explain the available experimental data on the translocation through the single-stranded mRNA, they are inconsistent with the experimental data on the translocation through the mRNA duplex; by contrast, the results obtained with Model II can explain quantitatively all of the available experimental data. With model II it is further shown that the efficient translocation induced by the binding of sparsomycin results mainly from the facilitation of the forward 30S head rotation, whereas the effect as the pawl plays only a little role. In addition, it is noted that the mRNA movement is brought about by the reverse intersubunit rotation rather than driven directly by the forward 30S head rotation, and the mechanism of antibiotic-induced translocation is similar to that of EF-G-catalyzed translocation.





Similar content being viewed by others
Notes
As discussed in detail before,55 available structural data showed that the ribosomes complexed with tRNAs in the classical non-rotated state always have no or nearly no 30S head rotation5,11,15,22–25,40,44,58. It is thus expected that during the translocation the ribosomal complex with no intersubunit rotation has the non-rotated 30S head, implying that the forward 30S head rotation can only take place in the rotated/hybrid state. Based on these structural data, it is also proposed that the transition from the rotated/hybrid state to the non-rotated state is accompanied or followed immediately by the reverse rotation of the 30S head if it is rotated. As shown before,55 with this proposal the theoretical data are in good agreement with the biochemical data.19
Biochemical data indicated that without a tRNA anticodon stem-loop bound to the 30S A site the translocation is inefficient even in the presence of EF-G.GTP, and only with a tRNA anticodon stem-loop bound to the 30S A site can the EF-G-catalyzed translocation become highly efficient.23 This could imply that the EF-G-catalyzed forward rotation of the 30S head is also mainly via the interaction of EF-G with the tRNA anticodon stem-loop bound to the 30S A site.
References
Blanchard, S. C., H. D. Kim, R. L. Gonzalez, Jr, J. D. Puglisi, and S. Chu. tRNA dynamics on the ribosome during translation. Proc. Natl Acad. Sci. USA 101:12893–12898, 2004.
Bulkley, D., C. A. Innis, G. Blaha, and T. A. Steitz. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc. Natl. Acad. Sci. USA 107:17158–17163, 2010.
Chen, J., A. Petrov, A. Tsai, S. E. O’Leary, and J. D. Puglisi. Coordinated conformational and compositional dynamics drive ribosome translocation. Nat. Struct. Mol. Biol. 20:718–727, 2013.
Cornish, P. V., D. N. Ermolenko, H. F. Noller, and T. Ha. Spontaneous intersubunit rotation in single ribosomes. Mol. Cell 30:578–588, 2008.
Dunkle, J. A., L. Wang, M. B. Feldman, et al. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332:981–984, 2011.
Dunkle, J. A., L. Xiong, A. S. Mankin, and J. H. Cate. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. 107:17152–17157, 2010.
Ermolenko, D. N., P. V. Cornish, T. Ha, and H. F. Noller. Antibiotics that bind to the A site of the large ribosomal subunit can induce mRNA translocation. RNA 19:158–166, 2013.
Ermolenko, D. N., and H. F. Noller. mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat. Struct. Mol. Biol. 18:457–463, 2011.
Fei, J., P. Kosuri, D. D. MacDougall, and R. L. Gonzalez, Jr. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol. Cell 30:348–359, 2008.
Feinberg, J. S., and S. Joseph. Identification of molecular interactions between P-site tRNA and the ribosome essential for translocation. Proc. Natl. Acad. Sci. USA 98:11120–11125, 2001.
Feng, S., Y. Chen, and Y.-G. Gao. Crystal structure of 70S ribosome with both cognate tRNAs in the E and P sites representing an authentic elongation complex. PLoS One 8:e58829, 2013.
Frank, J., H. Gao, J. Sengupta, N. Gao, and D. J. Taylor. The process of mRNA–tRNA translocation. Proc. Natl. Acad. Sci. USA 104:19671–19678, 2007.
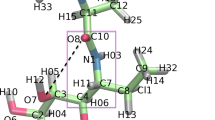
Fredrick, K., and H. F. Noller. Catalysis of ribosomal translocation by sparsomycin. Science 300:1159–1162, 2003.
Freier, S. M., R. Kierzek, J. A. Jaeger, N. Sugimoto, M. H. Caruthers, T. Neilson, and D. H. Tuener. Improved free-energy parameters for predictions of RNA duplex stability. Proc. Natl Acad. Sci. USA 83:9373–9377, 1986.
Gao, Y.-G., M. Selmer, C. M. Dunham, A. Weixlbaumer, A. C. Kelley, and V. Ramakrishnan. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326:694–699, 2009.
Gavrilova, L. P., O. E. Kostiashkina, V. E. Koteliansky, N. M. Rutkevitch, and A. S. Spirin. Factor free (“non-enzymic”) and factor-dependent systems of translation of polyuridylic acid by Escherichia coli ribosomes. J. Mol. Biol. 101:537–552, 1976.
Gavrilova, L. P., and A. S. Spirin. Stimulation of “non-enzymic” translocation in ribosomes by p-chloromercuribenzoate. FEBS Lett. 17:324–326, 1971.
Gromadski, K. B., and M. V. Rodnina. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol. Cell 13:191–200, 2004.
Guo, Z., and H. F. Noller. Rotation of the head of the 30S ribosomal subunit during mRNA translocation. Proc. Natl. Acad. Sci. USA 109:20391–20394, 2012.
Hansen, J. L., P. B. Moore, and T. A. Steitz. Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit. J. Mol. Biol. 330:1061–1075, 2003.
Hansen, J. L., T. M. Schmeing, P. B. Moore, and T. A. Steitz. Structural insights into peptide bond formation. Proc. Natl Acad. Sci. USA 99:11670–11765, 2002.
Jenner, L. B., N. Demeshkina, G. Yusupova, and M. Yusupov. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat. Struct. Mol. Biol. 17:555–560, 2010.
Joseph, S., and H. F. Noller. EF-G-catalyzed translocation of anticodon stem–loop analogs of transfer RNA in the ribosome. EMBO J. 17:3478–3483, 1998.
Korostelev, A., H. Asahara, L. Lancaster, et al. Crystal structure of a translation termination complex formed with release factor RF2. Proc. Natl. Acad. Sci. USA 105:19684–19689, 2008.
Laurberg, M., H. Asahara, A. Korostelev, J. Y. Zhu, S. Trakhanov, and H. F. Noller. Structural basis for translation termination on the 70S ribosome. Nature 454:852–857, 2008.
Lill, R., J. M. Robertson, and W. Wintermeyer. Binding of the 30-terminus of tRNA to 23S rRNA in the ribosomal exit site actively promotes translocation. EMBO J. 8:3933–3938, 1989.
Lin, J., M. G. Gagnon, D. Bulkley, and T. A. Steitz. Conformational changes of elongation factor G on the ribosome during tRNA translocation. Cell 160:219–227, 2015.
Liu, Q., and K. Fredrick. Contribution of intersubunit bridges to the energy barrier of ribosomal translocation. Nucleic Acids Res. 41:565–574, 2013.
Moazed, D., and H. F. Noller. Intermediate states in the movement of transfer RNA in the ribosome. Nature 342:142–148, 1989.
Mohan, S., J. P. Donohue, and H. F. Noller. Molecular mechanics of 30S subunit head rotation. Proc. Natl. Acad. Sci. USA 111:13325–13330, 2014.
Monro, R. E., M. L. Celma, and D. Vazques. Action of sparsomycin on ribosome-catalyzed peptidyl transfer. Nature 222:356–358, 1969.
Noller, H. F., M. M. Yusupov, G. Z. Yusupova, A. Baucom, and J. H. D. Cate. Translocation of tRNA during protein synthesis. FEBS Lett. 514:11–16, 2002.
Pestka, S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. VI. Oligopeptide synthesis and translocation on ribosomes in the presence and absence of soluble transfer factors. J. Biol. Chem. 244:1533–1539, 1969.
Ramrath, D. J., L. Lancaster, T. Sprink, T. Mielke, J. Loerke, H. F. Noller, and C. M. T. Spahn. Proc. Natl. Acad. Sci. USA 110:20964–20969, 2013.
Ratje, A. H., J. Loerke, A. Mikolajka, et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 468:713–716, 2010.
Rodnina, M. V., A. Savelsbergh, V. I. Katunin, and W. Wintermeyer. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature 385:37–41, 1997.
Savelsbergh, A., V. I. Katunin, D. Mohr, F. Peske, M. V. Rodnina, and W. Wintermeyer. An elongation factor G-induced ribosome rearrangement precedes tRNA–mRNA translocation. Mol. Cell 11:1517–1523, 2003.
Savelsbergh, A., N. B. Matassova, M. V. Rodnina, and W. Wintermeyer. Role of domains 4 and 5 in elongation factor G functions on the ribosome. J. Mol. Biol. 300:951–961, 2000.
Schuwirth, B. S., J. M. Day, C. W. Hau, G. R. Janssen, A. E. Dahlberg, J. H. D. Cate, and A. Vila-Sanjurjo. Structural analysis of kasugamycin inhibition of translation. Nat. Struct. Mol. Biol. 13:879–886, 2006.
Selmer, M., C. M. Dunham, F. V. Murphy, A. Weixlbaumer, S. Petry, A. C. Kelley, J. R. Weir, and V. Ramakrishnan. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313:1935–1942, 2006.
Shi, X., K. Chiu, S. Ghosh, and S. Joseph. Bases in 16S rRNA important for subunit association, tRNA binding, and translocation. Biochemistry 48:6772–6782, 2009.
Shoji, S., S. E. Walker, and K. Fredrick. Ribosomal translocation: one step closer to the molecular mechanism. ACS Chem. Boil. 4:93–107, 2009.
Southworth, D. R., J. L. Brunelle, and R. Green. EFG-independent translocation of the mRNA:tRNA complex is promoted by modification of the ribosome with Thiol-specific reagents. J. Mol. Biol. 324:611–623, 2002.
Stanley, R. E., G. Blaha, R. L. Grodzicki, M. D. Strickler, and T. A. Steitz. The structures of the anti-tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome. Nat. Struct. Mol. Biol. 17:289–293, 2010.
Takyar, S., R. P. Hickerson, and H. F. Noller. mRNA helicase activity of the ribosome. Cell 120:49–58, 2005.
Taylor, D. J., J. Nilsson, A. D. Merrill, G. R. Andersen, P. Nissen, and J. Frank. Structures of modified eEF2.80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J. 26:2421–2431, 2007.
Valle, M., A. Zavialov, J. Sengupta, U. Rawat, M. Ehrenberg, and J. Frank. Locking and unlocking of ribosomal motions. Cell 114:123–134, 2003.
Walker, S. E., S. Shoji, D. Pan, B. S. Cooperman, and K. Fredrick. Role of hybrid tRNA-binding states in ribosomal translocation. Proc. Natl. Acad. Sci. USA 105:9192–9197, 2008.
Wintermeyer, W., F. Peske, M. Beringer, K. B. Gromadski, A. Savelsbergh, and M. V. Rodnina. Mechanisms of elongation on the ribosome: dynamics of a macromolecular machine. Biochem. Soc. Trans. 32:733–737, 2004.
Xie, P. Dynamics of forward and backward translocation of mRNA in the ribosome. PLoS One 8:e70789, 2013.
Xie, P. Model of ribosome translation and mRNA unwinding. Eur. Biophys. J. 42:347–354, 2013.
Xie, P. Dynamics of tRNA translocation, mRNA translocation and tRNA dissociation during ribosome translation through mRNA secondary structures. Eur. Biophys. J. 43:229–240, 2014.
Xie, P. Origin of multiple intersubunit rotations before EF-G-catalyzed ribosomal translocation through the mRNA with a downstream secondary structure. BMC Biophys. 7:12, 2014.
Xie, P. Biphasic character of ribosomal translocation and non-Michaelis-Menten kinetics of translation. Phys. Rev. E 90:062703, 2014.
Xie, P. Model of ribosomal translocation coupled with intra- and inter-subunit rotations. Biochem. Biophys. Rep. 2:87–93, 2015.
Xie, P. Dwell-time distribution, long pausing and arrest of single-ribosome translation through the mRNA duplex. Int. J. Mol. Sci. 16:23723–23744, 2015.
Xie, P. A unified model of nucleic acid unwinding by the ribosome and the hexameric and monomeric DNA helicases. J. Theor. Biol. 380:359–366, 2015.
Yusupov, M. M., G. Z. Yusupova, A. Baucom, K. Lieberman, T. N. Earnest, J. H. D. Cate, and H. F. Noller. Crystal structure of the ribosome at 5.5 A resolution. Science 292:883–896, 2001.
Zavialov, A. V., and M. Ehrenberg. Peptidyl-tRNA regulates the GTPase activity of translation factors. Cell 114:113–122, 2003.
Zhou, J., L. Lancaster, J. P. Donohue, and H. F. Noller. Crystal structures of EF-G-ribosome complexes trapped in intermediate states of translocation. Science 340:1236086, 2013.
Zhou, J., L. Lancaster, J. P. Donohue, and H. F. Noller. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 345:1255030, 2014.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 11374352).
Conflict of Interest
Ping Xie declares that he has no conflicts of interest.
Ethical Standards
No human studies were carried out by the author for this article. No animal studies were carried out by the author for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor James L. McGrath oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Xie, P. Modeling Ribosomal Translocation Facilitated by Peptidyl Transferase Antibiotics. Cel. Mol. Bioeng. 9, 289–302 (2016). https://doi.org/10.1007/s12195-016-0433-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-016-0433-z