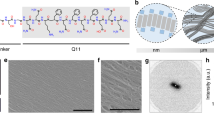
Abstract
Galectins are carbohydrate-binding proteins that act as extracellular signaling molecules in various normal and pathological processes. Galectin bioactivity is mediated by specific non-covalent interactions with cell-surface and extracellular matrix glycoproteins, which can enhance or inhibit signaling events that influence various cellular behaviors, including adhesion, proliferation, differentiation, and apoptosis. Here, we developed a materials approach to modulate galectin bioactivity by mimicking natural galectin–glycoprotein interactions. Specifically, we created a variant of a peptide that self-assembles into β-sheet nanofibers under aqueous conditions, QQKFQFQFEQQ (Q11), which has an asparagine residue modified with the monosaccharide N-acetylglucosamine (GlcNAc) at its N-terminus (GlcNAc-Q11). GlcNAc-Q11 self-assembled into β-sheet nanofibers under similar conditions as Q11. Nanofibrillar GlcNAc moieties were efficiently converted to the galectin-binding disaccharide N-acetyllactosamine (LacNAc) via the enzyme β-1,4-galactosyltransferase and the sugar donor UDP-galactose, while retaining β-sheet structure and nanofiber morphology. LacNAc-Q11 nanofibers bound galectin-1 and -3 in a LacNAc concentration-dependent manner, although nanofibers bound galectin-1 with higher affinity than galectin-3. In contrast, galectin-1 bound weakly to GlcNAc-Q11 nanofibers, while no galectin-3 binding to these nanofibers was observed. Galectin-1 binding to LacNAc-Q11 nanofibers was specific because it could be inhibited by excess soluble β-lactose, a galectin-binding carbohydrate. LacNAc-Q11 nanofibers inhibited galectin-1-mediated apoptosis of Jurkat T cells in a LacNAc concentration-dependent manner, but were unable to inhibit galectin-3 activity, consistent with galectin-binding affinity of the nanofibers. We envision that glycopeptide nanofibers capable of modulating galectin-1 bioactivity will be broadly useful as biomaterials for various medical applications, including cancer therapeutics, immunotherapy, tissue regeneration, and viral prophylaxis.







Similar content being viewed by others
References
Aida, T., E. W. Meijer, and S. I. Stupp. Functional supramolecular polymers. Science 335:813–817, 2012.
Arikawa, T., A. Matsukawa, K. Watanabe, K. M. Sakata, M. Seki, M. Nagayama, K. Takeshita, K. Ito, T. Niki, S. Oomizu, R. Shinonaga, N. Saita, and M. Hirashima. Galectin-9 accelerates transforming growth factor beta3-induced differentiation of human mesenchymal stem cells to chondrocytes. Bone 44:849–857, 2009.
Ban, L., and M. Mrksich. On-chip synthesis and label-free assays of oligosaccharide arrays. Angew. Chem. Int. Edit. 47:3396–3399, 2008.
Banh, A., J. Zhang, H. Cao, D. M. Bouley, S. Kwok, C. Kong, A. J. Giaccia, A. C. Koong, and Q. T. Le. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 71:4423–4431, 2011.
Baum, L. G., D. P. Blackall, S. Arias-Magallano, D. Nanigian, S. Y. Uh, J. M. Browne, D. Hoffmann, C. E. Emmanouilides, M. C. Territo, and G. C. Baldwin. Amelioration of graft versus host disease by galectin-1. Clin. Immunol. 109:295–307, 2003.
Baum, L. G., O. B. Garner, K. Schaefer, and B. Lee. Microbe–host interactions are positively and negatively regulated by galectin–glycan interactions. Front. Immunol. 5:284, 2014.
Beer, M. V., C. Rech, P. Gasteier, B. Sauerzapfe, J. Salber, A. Ewald, M. Moller, L. Elling, and J. Groll. The next step in biomimetic material design: poly-LacNAc-mediated reversible exposure of extra cellular matrix components. Adv. Healthc. Mater. 2:306–311, 2013.
Buskas, T., S. Ingale, and G. J. Boons. Glycopeptides as versatile tools for glycobiology. Glycobiology 16:113R–136R, 2006.
Camby, I., M. Le Mercier, F. Lefranc, and R. Kiss. Galectin-1: a small protein with major functions. Glycobiology 16:137R–157R, 2006.
Cedeno-Laurent, F., and C. J. Dimitroff. Galectin-1 research in T cell immunity: past, present and future. Clin. Immunol. 142:107–116, 2012.
Chan, J., K. O’Donoghue, M. Gavina, Y. Torrente, N. Kennea, H. Mehmet, H. Stewart, D. J. Watt, J. E. Morgan, and N. M. Fisk. Galectin-1 induces skeletal muscle differentiation in human fetal mesenchymal stem cells and increases muscle regeneration. Stem Cells 24:1879–1891, 2006.
Chen, J., R. R. Pompano, F. W. Santiago, L. Maillat, R. Sciammas, T. Sun, H. Han, D. J. Topham, A. S. Chong, and J. H. Collier. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 34:8776–8785, 2013.
Collier, J. H., and P. B. Messersmith. Enzymatic modification of self-assembled peptide structures with tissue transglutaminase. Bioconjug. Chem. 14:748–755, 2003.
Collier, J. H., J. S. Rudra, J. Z. Gasiorowski, and J. P. Jung. Multi-component extracellular matrices based on peptide self-assembly. Chem. Soc. Rev. 39:3413–3424, 2010.
Cortegano, I., V. del Pozo, B. Cardaba, B. de Andres, S. Gallardo, A. del Amo, I. Arrieta, A. Jurado, P. Palomino, F. T. Liu, and C. Lahoz. Galectin-3 down-regulates IL-5 gene expression on different cell types. J. Immunol. 161:385–389, 1998.
Du, X., J. Zhou, O. Guvench, F. O. Sangiorgi, X. Li, N. Zhou, and B. Xu. Supramolecular assemblies of a conjugate of nucleobase, amino acids, and saccharide act as agonists for proliferation of embryonic stem cells and development of zygotes. Bioconjug. Chem. 25:1031–1035, 2014.
Espelt, M. V., D. O. Croci, M. L. Bacigalupo, P. Carabias, M. Manzi, M. T. Elola, M. C. Munoz, F. P. Dominici, C. Wolfenstein-Todel, G. A. Rabinovich, and M. F. Troncoso. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization, and in vivo tumor growth. Hepatology 53:2097–2106, 2011.
Etulain, J., S. Negrotto, M. V. Tribulatti, D. O. Croci, J. Carabelli, O. Campetella, G. A. Rabinovich, and M. Schattner. Control of angiogenesis by galectins involves the release of platelet-derived proangiogenic factors. PLoS ONE 9:e96402, 2014.
Fortuna-Costa, A., A. M. Gomes, E. O. Kozlowski, M. P. Stelling, and M. S. Pavao. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol. 4:138, 2014.
Friedrichs, J., A. Manninen, D. J. Muller, and J. Helenius. Galectin-3 regulates integrin alpha2beta1-mediated adhesion to collagen-I and -IV. J. Biol. Chem. 283:32264–32272, 2008.
Galan, M. C., P. Dumy, and O. Renaudet. Multivalent glyco(cyclo)peptides. Chem. Soc. Rev. 42:4599–4612, 2013.
Galler, K. M., L. Aulisa, K. R. Regan, R. N. D’Souza, and J. D. Hartgerink. Self-assembling multidomain peptide hydrogels: designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J. Am. Chem. Soc. 132:3217–3223, 2010.
Garcia, I., A. Sanchez-Iglesias, M. Henriksen-Lacey, M. Grzelczak, S. Penades, and L. M. Liz-Marzan. Glycans as biofunctional ligands for gold nanorods: stability and targeting in protein-rich media. J. Am. Chem. Soc. 137:3686–3692, 2015.
Gasiorowski, J. Z., and J. H. Collier. Directed intermixing in multicomponent self-assembling biomaterials. Biomacromolecules 12:3549–3558, 2011.
Giano, M. C., D. J. Pochan, and J. P. Schneider. Controlled biodegradation of self-assembling beta-hairpin peptide hydrogels by proteolysis with matrix metalloproteinase-13. Biomaterials 32:6471–6477, 2011.
Godula, K., and C. R. Bertozzi. Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins. J. Am. Chem. Soc. 134:15732–15742, 2012.
Goldring, K., G. E. Jones, R. Thiagarajah, and D. J. Watt. The effect of galectin-1 on the differentiation of fibroblasts and myoblasts in vitro. J. Cell Sci. 115:355–366, 2002.
Goldstein, I. J., and R. D. Poretz. Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: The Lectins: Properties, Functions, and Applications in Biology and Medicine, edited by I. E. Liener, N. Sharon, and I. J. Goldstein. Orlando: Academic Press, 1986, pp. 103–115.
Hatano, K., K. Matsuoka, and D. Terunuma. Carbosilane glycodendrimers. Chem. Soc. Rev. 42:4574–4598, 2013.
Heusschen, R., A. W. Griffioen, and V. L. Thijssen. Galectin-9 in tumor biology: a jack of multiple trades. Biochim. Biophys. Acta 1836:177–185, 2013.
Horiguchi, N., K. Arimoto, A. Mizutani, Y. Endo-Ichikawa, H. Nakada, and S. Taketani. Galectin-1 induces cell adhesion to the extracellular matrix and apoptosis of non-adherent human colon cancer Colo201 cells. J. Biochem. 134:869–874, 2003.
Horlacher, T., M. A. Oberli, D. B. Werz, L. Krock, S. Bufali, R. Mishra, J. Sobek, K. Simons, M. Hirashima, T. Niki, and P. H. Seeberger. Determination of carbohydrate-binding preferences of human galectins with carbohydrate microarrays. ChemBioChem 11:1563–1573, 2010.
Hudalla, G. A., J. A. Modica, Y. F. Tian, J. S. Rudra, A. S. Chong, T. Sun, M. Mrksich, and J. H. Collier. A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv. Healthc. Mater. 2:1114–1119, 2013.
Hudalla, G. A., T. Sun, J. Z. Gasiorowski, H. Han, Y. F. Tian, A. S. Chong, and J. H. Collier. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat. Mater. 13:829–836, 2014.
Hughes, R. C. Galectins as modulators of cell adhesion. Biochimie 83:667–676, 2001.
Ingrassia, L., I. Camby, F. Lefranc, V. Mathieu, P. Nshimyumukiza, F. Darro, and R. Kiss. Anti-galectin compounds as potential anti-cancer drugs. Curr. Med. Chem. 13:3513–3527, 2006.
Ito, K., K. Stannard, E. Gabutero, A. M. Clark, S. Y. Neo, S. Onturk, H. Blanchard, and S. J. Ralph. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev. 31:763–778, 2012.
Jiang, H. R., Z. Al Rasebi, E. Mensah-Brown, A. Shahin, D. Xu, C. S. Goodyear, S. Y. Fukada, F. T. Liu, F. Y. Liew, and M. L. Lukic. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J. Immunol. 182:1167–1173, 2009.
Jung, J. H., M. Amaike, and S. Shinkai. Sol-gel transcription of novel sugar-based superstructures composed of sugar-integrated gelators into silica: creation of a lotus-shaped silica structure. Chem. Commun. 2343–2344, 2000.
Jung, J. P., A. K. Nagaraj, E. K. Fox, J. S. Rudra, J. M. Devgun, and J. H. Collier. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 30:2400–2410, 2009.
Kiyonaka, S., K. Sada, I. Yoshimura, S. Shinkai, N. Kato, and I. Hamachi. Semi-wet peptide/protein array using supramolecular hydrogel. Nat. Mater. 3:58–64, 2004.
Komatsu, H., S. Matsumoto, S. Tamaru, K. Kaneko, M. Ikeda, and I. Hamachi. Supramolecular hydrogel exhibiting four basic logic gate functions to fine-tune substance release. J. Am. Chem. Soc. 131:5580–5585, 2009.
Kuwabara, I., and F. T. Liu. Galectin-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 156:3939–3944, 1996.
Leffler, H., S. Carlsson, M. Hedlund, Y. Qian, and F. Poirier. Introduction to galectins. Glycoconj. J. 19:433–440, 2004.
Lei, C. X., W. Zhang, J. P. Zhou, and Y. K. Liu. Interactions between galectin-3 and integrinbeta3 in regulating endometrial cell proliferation and adhesion. Hum. Reprod. 24:2879–2889, 2009.
Levy, Y., R. Arbel-Goren, Y. R. Hadari, S. Eshhar, D. Ronen, E. Elhanany, B. Geiger, and Y. Zick. Galectin-8 functions as a matricellular modulator of cell adhesion. J. Biol. Chem. 276:31285–31295, 2001.
Li, X., Y. Kuang, J. Shi, Y. Gao, H. C. Lin, and B. Xu. Multifunctional, biocompatible supramolecular hydrogelators consist only of nucleobase, amino acid, and glycoside. J. Am. Chem. Soc. 133:17513–17518, 2011.
Li, X. M., Y. Kuang, and B. Xu. “Molecular trinity” for soft nanomaterials: integrating nucleobases, amino acids, and glycosides to construct multifunctional hydrogelators. Soft Matter 8:2801–2806, 2012.
Loo, Y., S. Zhang, and C. A. Hauser. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol. Adv. 30:593–603, 2012.
Lundquist, J. J., and E. J. Toone. The cluster glycoside effect. Chem. Rev. 102:555–578, 2002.
Martinez, A., C. Ortiz Mellet, and J. M. Garcia Fernandez. Cyclodextrin-based multivalent glycodisplays: covalent and supramolecular conjugates to assess carbohydrate-protein interactions. Chem. Soc. Rev. 42:4746–4773, 2013.
Miura, Y. Design and synthesis of well-defined glycopolymers for the control of biological functionalities. Polym. J. 44:679–689, 2012.
Moiseeva, E. P., E. L. Spring, J. H. Baron, and D. P. de Bono. Galectin 1 modulates attachment, spreading and migration of cultured vascular smooth muscle cells via interactions with cellular receptors and components of extracellular matrix. J. Vasc. Res. 36:47–58, 1999.
Motran, C. C., K. M. Molinder, S. D. Liu, F. Poirier, and M. C. Miceli. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur. J. Immunol. 38:3015–3027, 2008.
Nagae, M., N. Nishi, S. Nakamura-Tsuruta, J. Hirabayashi, S. Wakatsuki, and R. Kato. Structural analysis of the human galectin-9 N-terminal carbohydrate recognition domain reveals unexpected properties that differ from the mouse orthologue. J. Mol. Biol. 375:119–135, 2008.
Nagata, Y., and M. B. Burger. Wheat germ agglutinin: molecular characteristics and specificity for sugar binding. J. Biol. Chem. 249:3116–3122, 1974.
Oberg, C. T., H. Leffler, and U. J. Nilsson. Inhibition of galectins with small molecules. Chimia (Aarau) 65:18–23, 2011.
Pace, K. E., H. P. Hahn, and L. G. Baum. Preparation of recombinant human galectin-1 and use in T-cell death assays. Method Enzymol. 363:499–518, 2003.
Pieters, R. J. Inhibition and detection of galectins. ChemBioChem 7:721–728, 2006.
Rabinovich, G. A., and M. A. Toscano. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9:338–352, 2009.
Rabinovich, G. A., M. A. Toscano, S. S. Jackson, and G. R. Vasta. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 17:513–520, 2007.
Ramakrishnan, B., P. S. Shah, and P. K. Qasba. alpha-Lactalbumin (LA) stimulates milk beta-1,4-galactosyltransferase I (beta 4Gal-T1) to transfer glucose from UDP-glucose to N-acetylglucosamine. Crystal structure of beta 4Gal-T1 × LA complex with UDP-Glc. J. Biol. Chem. 276:37665–37671, 2001.
Rudra, J. S., Y. F. Tian, J. P. Jung, and J. H. Collier. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. USA 107:622–627, 2010.
Sansone, F., and A. Casnati. Multivalent glycocalixarenes for recognition of biological macromolecules: glycocalyx mimics capable of multitasking. Chem. Soc. Rev. 42:4623–4639, 2013.
Sauerzapfe, B., K. Krenek, J. Schmiedel, W. W. Wakarchuk, H. Pelantova, V. Kren, and L. Elling. Chemo-enzymatic synthesis of poly-N-acetyllactosamine (poly-LacNAc) structures and their characterization for CGL2-galectin-mediated binding of ECM glycoproteins to biomaterial surfaces. Glycoconj. J. 26:141–159, 2009.
Schattner, M., and G. A. Rabinovich. Galectins: new agonists of platelet activation. Biol. Chem. 394:857–863, 2013.
Shylaja, M., and H. S. Seshadri. Glycoproteins—an overview. Biochem. Educ. 17:170–178, 1989.
Solis, D., M. J. Mate, M. Lohr, J. P. Ribeiro, L. Lopez-Merino, S. Andre, E. Buzamet, F. J. Canada, H. Kaltner, M. Lensch, F. M. Ruiz, G. Haroske, U. Wollina, M. Kloor, J. Kopitz, J. L. Saiz, M. Menendez, J. Jimenez-Barbero, A. Romero, and H. J. Gabius. N-domain of human adhesion/growth-regulatory galectin-9: preference for distinct conformers and non-sialylated N-glycans and detection of ligand-induced structural changes in crystal and solution. Int. J. Biochem. Cell Biol. 42:1019–1029, 2010.
Stannard, K. A., P. M. Collins, K. Ito, E. M. Sullivan, S. A. Scott, E. Gabutero, I. Darren Grice, P. Low, U. J. Nilsson, H. Leffler, H. Blanchard, and S. J. Ralph. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 299:95–110, 2010.
Stillman, B. N., D. K. Hsu, M. Pang, C. F. Brewer, P. Johnson, F. T. Liu, and L. G. Baum. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176:778–789, 2006.
Stowell, S. R., C. M. Arthur, P. Mehta, K. A. Slanina, O. Blixt, H. Leffler, D. F. Smith, and R. D. Cummings. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 283:10109–10123, 2008.
Stowell, S. R., Y. Qian, S. Karmakar, N. S. Koyama, M. Dias-Baruffi, H. Leffler, R. P. McEver, and R. D. Cummings. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 180:3091–3102, 2008.
St-Pierre, C., H. Manya, M. Ouellet, G. F. Clark, T. Endo, M. J. Tremblay, and S. Sato. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 85:11742–11751, 2011.
Sturm, A., M. Lensch, S. Andre, H. Kaltner, B. Wiedenmann, S. Rosewicz, A. U. Dignass, and H. J. Gabius. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 173:3825–3837, 2004.
Thijssen, V. L., and A. W. Griffioen. Galectin-1 and -9 in angiogenesis: a sweet couple. Glycobiology 24:915–920, 2014.
Toscano, M. A., G. A. Bianco, J. M. Ilarregui, D. O. Croci, J. Correale, J. D. Hernandez, N. W. Zwirner, F. Poirier, E. M. Riley, L. G. Baum, and G. A. Rabinovich. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8:825–834, 2007.
Tribulatti, M. V., M. G. Figini, J. Carabelli, V. Cattaneo, and O. Campetella. Redundant and antagonistic functions of galectin-1, -3, and -8 in the elicitation of T cell responses. J. Immunol. 188:2991–2999, 2012.
Wan, S. Y., T. F. Zhang, and Y. Ding. Galectin-3 enhances proliferation and angiogenesis of endothelial cells differentiated from bone marrow mesenchymal stem cells. Transplant. Proc. 43:3933–3938, 2011.
Woolfson, D. N., and Z. N. Mahmoud. More than just bare scaffolds: towards multi-component and decorated fibrous biomaterials. Chem. Soc. Rev. 39:3464–3479, 2010.
Yang, R. Y., D. K. Hsu, L. Yu, H. Y. Chen, and F. T. Liu. Galectin-12 is required for adipogenic signaling and adipocyte differentiation. J. Biol. Chem. 279:29761–29766, 2004.
Yang, R. Y., G. A. Rabinovich, and F. T. Liu. Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 10:e17, 2008.
Yu, H., H. Chokhawala, R. Karpel, B. Wu, J. Zhang, Y. Zhang, Q. Jia, and X. Chen. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 127:17618–17619, 2005.
Zhou, M., A. M. Smith, A. K. Das, N. W. Hodson, R. F. Collins, R. V. Ulijn, and J. E. Gough. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 30:2523–2530, 2009.
Acknowledgments
This research was supported by the National Institutes of Health (NIBIB, 1R01EB009701; NCI, U54 CA151880; NIAID, 1F32AI096769) and the National Science Foundation (DMR-1455201). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and BioEngineering, the National Institute of Allergy and Infectious Disease, the National Cancer Institute, the National Institutes of Health, or the National Science Foundation. MALDI-TOF was performed in the University of Chicago Mass Spectrometry facility and the University of Florida Mass Spectrometry facility, with support from NSF CHE MRI 1040016. CD was performed in the University of Chicago Biophysics Core. TEM was performed in the University of Chicago Materials Research Center.
Conflict of interest
Antonietta Restuccia, Ye F. Tian, Joel H. Collier, and Gregory A. Hudalla declare that they have no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Michael R. King oversaw the review of this article.
This paper is designated as a 2014 BMES Outstanding Contribution.
Rights and permissions
About this article
Cite this article
Restuccia, A., Tian, Y.F., Collier, J.H. et al. Self-Assembled Glycopeptide Nanofibers as Modulators of Galectin-1 Bioactivity. Cel. Mol. Bioeng. 8, 471–487 (2015). https://doi.org/10.1007/s12195-015-0399-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-015-0399-2