Abstract
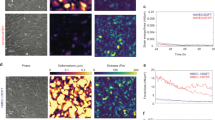
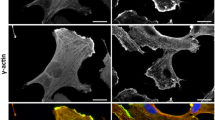
Vascular endothelial growth factor (VEGF) induces endothelial cell proliferation, migration, and a host of cellular responses that are necessary for angiogenesis. The effects of VEGF on gene expression in endothelial cells have been well characterized, and many of the chemical pathways have been elucidated. Similarly, VEGF is known to impact cytoskeletal structure and cellular motility. Here, we show that VEGF influences cytoskeletal-nuclear interaction by imaging human umbilical vein endothelial cells treated with VEGF. We visualize increased actin fibers present at the nucleus in VEGF treated cells. We also quantify increased fluctuations at the nuclear envelope with VEGF-treatment. This manifests itself as both a gross nuclear fluctuations, quantifiable as changes in nuclear area, and fine fluctuations, which can be visualized over several hours. Further, we observe an increase in intranuclear velocity of chromatin-bound probes. Our findings suggest enhanced mechanical coupling between the cytoskeleton and nucleus under VEGF treatment, which may have implications in the mechanobiology of altered gene expression during angiogenesis.





Similar content being viewed by others
References
Abe, M., and Y. Sato. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis 4:289–298, 2001.
Booth-Gauthier, E. A., T. A. Alcoser, G. Yang, and K. N. Dahl. Force-induced changes in subnuclear movement and rheology. Biophys. J. 103:2423–2431, 2012.
Chambliss, A. B., et al. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci. Rep. 3:1087, 2013.
Chancellor, T. J., J. Lee, C. K. Thodeti, and T. Lele. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 99:115–123, 2010.
Dahl, K. N., and A. Kalinowski. Nucleoskeleton mechanics at a glance. J. Cell Sci. 124:675–678, 2011.
Dahl, K. N., A. J. Ribeiro, and J. Lammerding. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 102:1307–1318, 2008.
Dalous, J., et al. Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation. Biophys. J. 94:1063–1074, 2008.
Ghosh, D., et al. Integral role of platelet-derived growth factor in mediating transforming growth factor-beta1-dependent mesenchymal stem cell stiffening. Stem Cells Dev. 23:245–261, 2013.
Jamali, T., Y. Jamali, M. Mehrbod, and M. R. Mofrad. Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Int. Rev. Cell Mol. Biol. 287:233–286, 2011.
Khatau, S. B., D. H. Kim, C. M. Hale, R. J. Bloom, and D. Wirtz. The perinuclear actin cap in health and disease. Nucleus 1:337–342, 2010.
Khatau, S. B., et al. A perinuclear actin cap regulates nuclear shape. Proc. Natl Acad. Sci. U.S.A. 106:19017–19022, 2009.
Khatau, S. B., et al. The differential formation of the LINC-mediated perinuclear actin cap in pluripotent and somatic cells. PLoS ONE 7:e36689, 2012.
Lombardi, M. L., et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 286:26743–26753, 2011.
Lovett, D. B., N. Shekhar, J. A. Nickerson, K. J. Roux, and T. P. Lele. Modulation of nuclear shape by substrate rigidity. Cell. Mol. Bioeng. 6:230–238, 2013.
Luxton, G. W., E. R. Gomes, E. S. Folker, H. J. Worman, and G. G. Gundersen. TAN lines: a novel nuclear envelope structure involved in nuclear positioning. Nucleus 2:173–181, 2011.
Mellad, J. A., D. T. Warren, and C. M. Shanahan. Nesprins LINC the nucleus and cytoskeleton. Curr. Opin. Cell Biol. 23:47–54, 2011.
Misteli, T. Beyond the sequence: cellular organization of genome function. Cell 128:787–800, 2007.
Morgan, J. T., et al. Nesprin-3 regulates endothelial cell morphology, perinuclear cytoskeletal architecture, and flow-induced polarization. Mol. Biol. Cell 22:4324–4334, 2011.
Rivera, C. G., S. Mellberg, L. Claesson-Welsh, J. S. Bader, and A. S. Popel. Analysis of VEGF—a regulated gene expression in endothelial cells to identify genes linked to angiogenesis. PLoS ONE 6:e24887, 2011.
Roukos, V., B. Burman, and T. Misteli. The cellular etiology of chromosome translocations. Curr. Opin. Cell Biol. 25:357–364, 2013.
Rousseau, S., F. Houle, and J. Huot. Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc. Med. 10:321–327, 2000.
Rousseau, S., et al. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J. Biol. Chem. 275:10661–10672, 2000.
Sato, Y. et al. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann. N. Y. Acad. Sci. 902:201–205; discussion 205–207 (2000).
Starr, D. A. A nuclear-envelope bridge positions nuclei and moves chromosomes. J. Cell Sci. 122:577–586, 2009.
van der Meer, A. D., K. Vermeul, A. A. Poot, J. Feijen, and I. Vermes. A microfluidic wound-healing assay for quantifying endothelial cell migration. Am. J. Physiol. Heart Circ. Physiol. 298:H719–H725, 2010.
Weston, G. C., I. Haviv, and P. A. Rogers. Microarray analysis of VEGF-responsive genes in myometrial endothelial cells. Mol. Hum. Reprod. 8:855–863, 2002.
Yang, G., L. A. Cameron, P. S. Maddox, E. D. Salmon, and G. Danuser. Regional variation of microtubule flux reveals microtubule organization in the metaphase meiotic spindle. J. Cell Biol. 182:631–639, 2008.
Yang, G., Matov, A., and Danuser, D. In: IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05) (San Diego, CA, 2005).
Yang, M. T., D. H. Reich, and C. S. Chen. Measurement and analysis of traction force dynamics in response to vasoactive agonists. Integr. Biol. (Camb) 3:663–674, 2011.
Acknowledgments
We acknowledge G. Yang (Carnegie Mellon University, Department of Biomedical Engineering) for aid in image analysis development, J. Szymanski and A. Feinberg (Carnegie Mellon University, Department of Biomedical Engineering) for help with confocal imaging, and S. Kumar (UC Berkeley, Department of Bioengineering) for reagents. This work is supported by the NSF (NSF-CBET-0954421, CMMI-1300476 to KND) and CMU fellowship support to STS (ARCS, Bertucci, and Meade).
Conflict of interest
Stephen T. Spagnol, James S. Weltz, Yongqiang Xue, and Kris Noel Dahl declare that they have no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Stephen T. Spagnol, James S. Weltz, and Yongqiang Xue contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (AVI 281 kb)
Supplementary material 3 (AVI 263 kb)
Supplementary material 4 (AVI 232 kb)
Supplementary material 5 (AVI 249 kb)
Supplementary material 6 (AVI 14495 kb)
Rights and permissions
About this article
Cite this article
Spagnol, S.T., Weltz, J.S., Xue, Y. et al. Mechanical Coupling of the Endothelial Cytoskeleton and Nucleus with VEGF Stimulation. Cel. Mol. Bioeng. 7, 225–230 (2014). https://doi.org/10.1007/s12195-014-0327-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0327-x