Abstract
Folding of the myosin head often requires the joint actions of Hsp90 and a dedicated UNC45, Cro1, She4 (UCS) domain-containing cochaperone protein. Relatively weak sequence conservation exists between the single UCS protein of simple eukaryotes (She4 in budding yeast) and the two UCS proteins of higher organisms (the general cell and smooth muscle UNC45s; UNC45-GC and UNC45-SM respectively). In vertebrates, UNC45-GC facilitates cytoskeletal function whereas the 55% identical UNC45-SM assists in the assembly of the contractile apparatus of cardiac and skeletal muscles. UNC45-SM, unlike UNC45-GC, shares with yeast She4 an IDSL sequence motif known to be a site of in vivo serine phosphorylation in yeast. Investigating this further, we found that both a non-phosphorylatable (S18A) and a phosphomimetic (S18E) mutant form of She4 could rescue the type 1 myosin localisation and endocytosis defects of the yeast she4Δ mutant at 39 °C. Nevertheless, at higher temperature (45 °C), only She4 (S18A), not She4(S18E), could substantially rescue the cell lysis defect of she4Δ mutant cells. In the yeast two-hybrid system, the non-phosphorylatable S18A and S251A mutant forms of She4 and UNC45-SM still displayed the stress-enhanced in vivo interaction with Hsp90 seen with the wild-type She4 and UNC45-SM. Such high-temperature enforcement to interaction was though lost with the phosphomimetic mutant forms (She4(S18E) and UNC45-SM (S251E)), an indication that phosphorylation might suppress these increases in She4/Hsp90 and UNC45-SM/Hsp90 interaction with stress.
Similar content being viewed by others
Introduction
Vertebrates have two forms of cytosolic Hsp90, Hsp90α and Hsp90β. There is evidence that their functions are not completely identical. In many tissues, the stress-induced isoform is Hsp90α whereas Hsp90β is more usually constitutively expressed and seems to be associated with development, long-term cell adaptation and evolution (Sreedhar et al. 2004). In mice, Hsp90β is essential for embryonic development, while the loss of Hsp90α is fully compatible with viability though it causes a block to spermatogenesis (Grad et al. 2011). Zebrafish studies have identified Hsp90α as being highly expressed in striated muscle while Hsp90β predominates in other tissues (Krone et al. 2003). Furthermore, as described below, Hsp90α and Hsp90β also differ in their association with UNC45, Cro1 and She4 (UCS) proteins, cochaperones that cooperate with Hsp90 during the folding of the myosin head.
The importance of UCS proteins was initially apparent from the study of Caenorhabditis elegans UNC-45 (“UNCoordinated”) mutants. These characteristically display defects in both motility (Barral et al. 1998; Ao and Pilgrim 2000) and cytokinesis during embryogenesis (Kachur et al. 2004). The single C. elegans UNC45 protein was subsequently shown to associate with both Hsp90 and myosin (Barral et al. 2002), its action facilitating not just myosin folding but also the regulation of myosin levels by targeting excess or damaged myosin to the proteasome for degradation (Landsverk et al. 2007). C. elegans UNC45 has been shown to form linear multimers, a filament assembly scaffold for the direct coupling of myosin folding with myofilament formation and the organisation of sarcomeric repeats (Gazda et al. 2013).
The sole UCS protein of budding yeast, She4, is required for actin cytoskeleton polarisation, endocytosis (Wendland et al. 1996) and the asymmetric messenger RNA (mRNA) localisation of ASH1 mRNA to daughter cells (Long et al. 1997). She4 interacts with the Saccharomyces cerevisiae class I myosins (Myo3 and Myo5) in a temperature-dependent manner (Toi et al. 2003; Wesche et al. 2003). Rng3, the UCS protein of the fission yeast Schizosaccharomyces pombe, interacts with both Hsp90 (Mishra et al. 2005) and the class II myosin Myo2 (Lord and Pollard 2004; Mishra et al. 2005), increasing myosin II affinity for actin filaments (Lord et al. 2008). RNG3 mutants show a 10-fold decrease in Myo5 levels, a 4-fold decrease in cortical actin patches (Lord et al. 2008) and defective cytokinesis (Wong et al. 2000). Thus, while the phenotypes associated with mutations in the C. elegans and fungal UCS proteins would seem—at first sight—to differ considerably, all relate to effects on myosin assembly and/or function.
Fungi and worms have just this single UCS protein. However, vertebrates have two—a general cell UNC45-GC (or Unc-45A) expressed in most somatic cells and a striated muscle UNC45-SM (Unc-45B) highly expressed only in the heart and skeletal muscle (Hutagalung et al. 2002; Price et al. 2002). Zebrafish studies have revealed that these UNC45s are not functionally redundant (Comyn and Pilgrim 2012). UNC45-GC plays a role in the cytoskeletal functions of most cells while UNC45-SM has a more specific role in the assembly of the contractile apparatus of cardiac and skeletal muscles (reviewed in Ni and Odunuga 2014; Lee et al. 2014). Furthermore, UNC45-SM associates specifically with Hsp90α, not Hsp90β (Etard et al. 2007). In contrast, UNC45-GC interacts preferentially with Hsp90β in vitro, mediating Hsp90β but not Hsp90α function when chaperoning the progesterone receptor to its hormone-binding state (Chadli et al. 2008).
UCS proteins are characterised by a C-terminal UCS domain containing several beta-catenin-like repeat sequences (Fig. 1). Indications of how this domain might facilitate the association of the myosin head with its actin filament binding site have emerged from the atomic structure of the yeast She4 dimer (Shi and Blobel 2010). UCS proteins also have a central domain of less defined function and—in the case of the vertebrate and worm proteins—an N-terminal tetratricopeptide (TPR) repeat (Fig. 1). The fungal UCS proteins lack this latter TPR domain, yet they still interact with Hsp90 (Millson et al. 2004; Mishra et al. 2005). Screening for protein phosphorylations in S. cerevisiae has revealed that She4 is phosphorylated on Ser18 (Albuquerque et al. 2008). This serine lies within a short IDSL sequence motif that is also to be found in the human UNC45-SM, though not UNC45-GC (Fig. 1). While this motif is not present in many fungal UNC45s, also the C. elegans UNC45, it appears relatively conserved in vertebrates (in the zebrafish UNC45-SM it is NDSL(251–255)). We have considered whether this Ser18 phosphorylation might regulate the function of the S. cerevisiae She4. Already, there are precedents for the phosphorylation of cochaperones of the Hsp90 system facilitating the “loading” of client proteins onto the chaperone complex. Phosphorylation, then dephosphorylation, of Cdc37 is needed for the efficient presentation of nascent protein kinases to Hsp90 (Mandal et al. 2007; Vaughan et al. 2008). In addition, a Ser361 phosphorylation of Sgt1 inhibits the dimerisation of this Sgt, a dimerisation needed for Sgt1 and Hsp90 to assist the binding of Skp1 to the yeast kinetochore (Bansal et al. 2009). This report describes an investigation into the effects of mutating Ser18 of She4 in vivo in yeast.
Materials and methods
Yeast strains and culture
The strains generated for this study are listed in Table 1. DNA cassettes for gene deletion were generated by PCR using hphMX4 (Goldstein and McCusker 1999) as template, and cassettes for C-terminal tagging of genes with green fluorescent protein (GFP) were generated using pUG23 (Niedenthal et al. 1996) as template.
Engineering yeast for the expression of mutant She4 under native promoter control
SHE4 sequences were first PCR-amplified from yeast genomic DNA using primers which add a 6xHis-tag at the N-terminus of the gene, then inserted into pCR®-XL-TOPO® (Invitrogen), thus providing the template for the site-directed mutagenesis of Ser18 using the QuickChange Mutagenesis Kit (Stratagene) (primer sequences available on request).
After sequence confirmation, these pCR®-XL-TOPO clones containing the genes for either wild-type, S18A or S18E 6xHis-SHE4 were PCR-amplified using primers GAAAAGATTACTAAAAAATTAGAATCACGACTAGTATGCATCATCATCATCATCATCCACTGTGTGAGAAAGGGA (His-tag sequence underlined) and TCCATAGAATTCCTGCAGCCCGGGGGATCCACTAGTTTAGACTTTAATTTTAGCAAG—these having 3′ sequence homologies to the SHE4-coding region and 5′ homologies (italicised) to the sequence of pShep next to the SpeI restriction site of the latter plasmid. pShep, constructed for this study, is a centromeric URA3 plasmid designed for expressing genes under the control of SHE4 promoter and terminator sequences. It comprises pRS416 (Sikorski and Hieter 1989) with inserts of the native SHE4 promoter (−520 to −1) between its Not1 and Spe1 sites and the SHE4 terminator region (+2971 to +3378) between its EcoR1 and Sal1 sites.
The above PCR-amplified genes for either the wild-type, S18A or S18E 6xHis-She4 were next inserted into yeast by homologous recombination, transforming BY4741 she4∆ (Table 1) to uracil prototrophy with the appropriate PCR product and SpeI-cleaved pShep. Transformants were initially checked by colony PCR, then protein extracts were analysed by western blotting using an anti-His antibody to confirm similar levels of 6xHis-tagged protein expression.
Phenotype analysis
Rescue of endocytosis at high temperature was analysed as the uptake of FM4–64, a fluorescent lipophilic styryl dye which stains the vacuole via the endocytic pathway (Smythe and Ayscough 2006). Cells growing on minus uracil dropout medium at 30 °C were incubated with 16 μM FM4–64 (Invitrogen, Ltd.) for 10 min, then chased in the absence of dye for 45 min at 30 or 39 °C. Samples were observed by fluorescence microscopy, acquiring images with the same exposure parameters so as to allow a direct comparison of image intensities.
For heat survival measurements, cultures growing on minus uracil dropout medium at 30 °C were incubated 1 h at 45 °C, survival being measured by plating dilutions on YPD agar plates before and after the 45 °C treatment and counting colonies after 3 days at 30 °C.
Yeast two-hybrid analysis
UNC45-SM was PCR-amplified from its cDNA (image clone 40008187, Geneservice, Cambridge, UK), then inserted into pCR®-XL-TOPO® prior to site-directed mutagenesis of Ser251 using the QuickChange Mutagenesis Kit. After sequence confirmation, the full-length wild-type, S251A and S251E UNC45-SM genes, as well as the above wild-type, S18A and S18E genes for yeast SHE4, were PCR-amplified using primers that add the terminal sequences for homologous recombination with linearised plasmids pADC or pBDC (as detailed in Millson et al. 2005). These PCR products were then transformed, together with the Y2H vectors pADC or pBDC, into the yeast strains used for yeast two-hybrid (Y2H) screening in our earlier studies, PJ69-4a or PJ69-4α respectively (Millson et al. 2003, 2004, 2005). After confirming that the resulting fusions did not self-activate in these PJ69-4 cells (did not appreciably enhance basal expression from the GAL4 promoter-directed β-galactosidase gene), these transformants were next mated with PJ69-4 cells of the opposite mating type, cells that express either Hsp82-BD, Hsp90α-BD or AD-Myo5. Quantitative analysis of Y2H interaction strength, GAL4 promoter-directed β-galactosidase gene expression, was as previously described (Millson et al. 2003, 2004, 2005). Control cells were those containing the appropriate pBDC fusion and empty pADC, since basal expression in this system is generally due to the latter plasmid (Millson et al. 2004).
Results
Lack of She4 causes defective Myo5 localisation and endocytosis at 39 °C
In this study, the functionality of She4 in yeast was studied as its ability, when expressed in cells of the she4Δ mutant, to rescue (i) the defective localisation of the class I myosin Myo5—observed as a functional Myo5-GFP fusion—at 39 °C, (ii) the defective localisation of the fluorescent dye FM4-64 to the vacuolar membrane, also at 39 °C and (iii) cell survival at 45 °C. FM4-64 is a lipophilic styryl dye that is normally rapidly internalised in budding yeast through endocytosis, whereupon it is subsequently targeted to the vacuolar membrane (Smythe and Ayscough 2006).
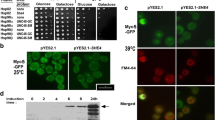
Myo5, one of the two class I myosins of overlapping function in S. cerevisiae (Myo3/5), plays an important role in endocytosis. Whereas no phenotype is apparent with the loss of either these myosins individually, a double myo3Δ myo5Δ knockout exhibits severe defects in both growth and the organisation of the actin cytoskeleton (Goodson et al. 1996). We confirmed that Myo5 is substantially functional with a C-terminal GFP tag by generating the strain BY4741 MYO5-GFP myo3Δ (Table 1). This did not display the severe phenotype described for the double myo3Δ myo5Δ knockout, but instead displayed normal growth and Myo5-GFP localisation to cortical patches at 39 °C. Similarly, She4 is also substantially functional when tagged with GFP, since the BY4741 SHE4-GFP strain (Table 1) did not exhibit she4Δ mutant phenotypes (unpublished observations).
In yeast, She4 appears especially important for the functioning of class I myosins when cells are subject to stress, since the phenotypes of the she4Δ mutant are most apparent at high temperature (Toi et al. 2003; Wesche et al. 2003). We analysed the localisation of Myo5-GFP and FM4-64 in strains BY4741 MYO5-GFP and BY4741 MYO5-GFP she4Δ (Table 1), both at 25 °C and in cells subjected to a 39 °C heat shock (Fig. 2a). At the lower temperature, both strains showed Myo5-GFP localisation to cortical patches as observed in earlier studies (Goodson et al. 1996; Toi et al. 2003; Wesche et al. 2003). However, in the cells lacking She4, more of this Myo5-GFP was dispersed through the cytosol, especially at 39 °C when patch-like localisation of the Myo5-GFP was almost completely lost (Fig. 2a). This reflects the action of She4 in facilitating the localisation of Myo5, especially at high temperature. We also found a She4-GFP fusion to be substantially localised to actin cortical patches at 39 °C (Fig. 2b).
a Myo5-GFP localisation and FM4-64 staining in BY4741 MYO5-GFP wild-type (WT) or she4Δ cells either in growth at room temperature (25 °C) or heat shocked from 25 to 39 °C for 1 h. b BY4741 SHE4-GFP cells growing at 39 °C were fixed and stained with rhodamine-phalloidin to observe co-localisation with actin. Scale bar 5 μm
Another prominent feature of the she4Δ mutant is its defect in endocytosis, apparent from the relatively weak FM4-64 staining of the vacuole at 39 °C in Fig. 2a.
The in vivo effects of She4(S18A) and She4(S18E) expression
Genes for wild-type as well as S18A and S18E mutant forms of 6xHis-She4 were inserted into strain BY4741 MYO5-GFP she4Δ (Table 1), these genes being under the control of the native SHE4 gene promoter and terminator sequences and carried on a centromeric plasmid vector (see Materials and methods). Both Myo5-GFP and FM4-64 staining in the resultant she4Δ cells containing either empty pShep vector, the non-mutant 6xHis-She4 or S18A and S18E mutant forms of 6xHis-She4 was then analysed (Fig. 3).
All three (wild type, S18E and S18A) forms of 6xHis-She4 were observed to rescue Myo5-GFP movement from the cytosol to organised patches in she4Δ cells at 39 °C (Fig. 3). Analysing FM4-64, all three forms of 6xHis-She4 were also found to have facilitated the targetting of the FM4-64 endocytic marker to the vacuole (Fig. 3). Therefore, both 6xHis-She4(S18E) and 6xHis-She4(S18A) were able to provide substantial rescue of the defective endocytosis in these she4Δ cells at 39 °C (Fig. 2a).
One can be reasonably confident that the above analysis relates to viable cells since 39 °C—the maximum growth temperature of BY4741 cells—represents a supraoptimal, yet sublethal heat stress, conditions under which the she4Δ mutant shows no enhanced loss of viability. Also, the cells observed here were only subjected to this temperature for less than 2 h during the microscopy. We noted, however, that the she4Δ mutant exhibits a strong phenotype of cell lysis at more stressful temperatures (Fig. 4a), losing viability much more rapidly than the wild type. Expressions of both the wild type and the non-phosphorylatable S18A mutant form of 6xHis-She4 were found to restore viability to she4Δ cells maintained at 45 °C, whereas the expression of the phosphomimetic mutant 6xHis-She4(S18E) provided little rescue of viability under the same conditions (Fig. 4b). This is an indication that the S18 phosphorylation of She4 may act to modulate the operation of this myosin-specific cochaperone (see Discussion).
Yeast two hybrid analysis
The effects of stress on the in vivo interactions of She4 can be analysed using the yeast two-hybrid (Y2H) system. In this system, the Gal4 activator domain (AD)-She4 fusion displays a temperature-reinforced interaction in vivo with the Hsp82-Gal4 DNA-binding domain (BD) fusion (Millson et al. 2004), the latter a functional form of Hsp90 chaperone (Millson et al. 2003). We investigated whether the S18A and S18E mutant forms of AD-She4 would still display this interaction. As shown in Fig. 5a, the non-phosphorylatable She4(S18A) showed an increased Hsp90 interaction with heat shock in this Y2H system that was comparable to that displayed by the wild-type She4. In contrast, the phosphomimetic mutant She4(S18E) showed no appreciable increase in its Y2H interaction with Hsp90 at 39 °C (Fig. 5a). This is an indication that the Ser18 phosphorylation of She4 might be acting to suppress any reinforcement of She4/Hsp90 binding upon stress.
Y2H interactions measured at 25 °C (−) and 39 °C (+). a Hsp82-BD with She4 WT, S18A and S18E. b Hsp90α-BD with WT, S251E and S251A mutant forms of human AD-UNC45-SM. c AD-Myo5 with She4-BD WT, S18A and S18E. d AD-Myo5 with WT, S251E and S251A mutant forms of human BD-UNC45-SM. Data shown are the mean and SD of eight replicate assays from the same cell culture
In the light of this result, we also analysed in the Y2H system the corresponding wild-type, S251A and S251E mutant forms of the human UNC45-SM, studying their interaction with the human Hsp90α (in zebrafish, UNC45-SM appears to associates specifically with the Hsp90α isoform of Hsp90; Etard et al. 2007). As with the corresponding native yeast proteins, Y2H interaction of the human Hsp90α with the wild-type and non-phosphorylatable S251A mutant UNC45-SM was markedly enhanced when the Y2H strains were exposed to heat stress (Fig. 5b). However, the phosphomimetic S251E mutant form of UNC45-SM largely lacked any stress reinforcement of this interaction (Fig. 5b), a result even more marked than that obtained with the corresponding phosphomimetic (S18E) mutant form of She4 (Fig. 5a).
We also analysed how these mutations in She4 and UNC45-SM affect the Y2H interaction with yeast type 1 myosin, Myo5 (Fig. 5c, d). These interactions, modestly enhanced by stress, appeared stronger with the S18A and S251A mutant forms of She4 and UNC45-SM than with the corresponding phosphomimetic S18E and S251E mutant forms of these two UCS proteins (Fig. 5c, d).
Discussion
Yeast She4 is known to be phosphorylated on Ser18 in vivo (Albuquerque et al. 2008). This serine lies within a short sequence motif (IDSL; Fig. 1) which is also present in the human UNC45-SM, despite the limited sequence conservation between these UCS proteins of yeast and man. This report describes an investigation into the in vivo effects in yeast of mutating this Ser18 residue of She4 to either a non-phosphorylatable alanine or a phosphomimetic glutamic acid, as well as the effects on in vivo interactions in the Y2H system of similarly mutating this Ser18 in yeast She4 and the corresponding Ser251 in the IDSL motif of the human UNC45-SM.
Fluorescence microscopic analysis of Myo5-GFP and FM4-64 revealed that the S18E and S18A mutant forms of 6xHis-She4 were still able to provide substantial rescue of the type 1 myosin localization and endocytosis defects apparent in she4Δ cells at 39 °C (Fig. 3). It was at a higher, more stressful temperature—conditions where she4Δ cells normally lyse—that our study uncovered the strongest phenotypic difference for the cells expressing the wild-type and (S18A) mutant 6xHis-She4 as compared to those expressing 6xHis-She4(S18E). Only 6xHis-She4(S18A) could provide the rescue of she4Δ cell viability at high temperature seen with the expression of the wild-type 6xHis-She4 (Fig. 4b). This may relate to the Y2H analysis, where it was evident that She4(S18E) had lost the capacity to engage in a temperature-enhanced interaction in vivo with Hsp90, a reinforcement that was still displayed by the non-phosphorylatable She4(S18A) (Fig. 5a). The Y2H interactions of the corresponding human proteins, UNC45-SM/Hsp90α and UNC45-SM(251A)/Hsp90α, show even greater reinforcement as the Y2H strains are exposed to high temperature, but again this was lost with the corresponding phosphomimic mutant form of UNC45-SM (UNC45-SM(251E)/Hsp90α; Fig. 5b).
No evidence has yet emerged to suggest that protein phosphorylation plays a role in UCS protein action. Only at 45 °C were we able to identify a strong phenotypic difference between the cells expressing 6xHis-She4(S18E) as compared to those with 6xHis-She4(S18A) (Fig. 4b). This S18E mutation also compromises the stress reinforcement to the interaction with Hsp90 in the Y2H system (Fig. 5a). In conclusion, our study indicates that Ser18 phosphorylation of She4 is non-essential for She4 to facilitate Myo5 localisation and endocytosis at 39 °C (Fig. 4a), yet also reveals that the S18E mutant form of She4 is not devoid of phenotype. The latter finding suggests that the phosphorylation/dephosphorylation of Ser18 might be involved in the fine tuning of She4 action and become especially important at extreme temperatures, conditions under which She4 is essential for cell viability (Fig. 4).
Abbreviations
- UCS protein:
-
A protein that contains the domain shared by UNC45, Cro1 and She4
- TPR:
-
Tetratricopeptide
- GFP:
-
Green fluorescent protein
- BD:
-
Gal4 DNA-binding domain
- AD:
-
Gal4 activator domain
- Y2H:
-
Yeast two-hybrid
References
Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteomics 7:1389–1396
Ao W, Pilgrim D (2000) Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J Cell Biol 148:375–384
Bansal PK, Nourse A, Abdulle R, Kitagawa K (2009) Sgt1 dimerization is required for yeast kinetochore assembly. J Biol Chem 284:3586–3592
Barral JM, Bauer CC, Ortiz I, Epstein HF (1998) Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J Cell Biol 143:1215–1225
Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF (2002) Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295:669–671
Chadli A, Felts SJ, Toft DO (2008) GCUNC45 is the first Hsp90 co-chaperone to show alpha/beta isoform specificity. J Biol Chem 283:9509–9512
Comyn SA, Pilgrim D (2012) Lack of developmental redundancy between Unc45 proteins in zebrafish muscle development. PLoS One 7:e48861
Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strahle U (2007) The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol 308:133–143
Gazda L, Pokrzywa W, Hellerschmied D et al (2013) The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans. Cell 152:183–195
Goldstein AL, McCusker JH (1999) Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541–1553
Goodson HV, Anderson BL, Warrick HM, Pon LA, Spudich JA (1996) Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J Cell Biol 133:1277–1291
Grad I, Cederroth CR, Walicki J et al (2011) The molecular chaperone Hsp90alpha is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One 5:e15770
Hutagalung AH, Landsverk ML, Price MG, Epstein HF (2002) The UCS family of myosin chaperones. J Cell Sci 115:3983–3990
Kachur T, Ao W, Berger J, Pilgrim D (2004) Maternal UNC-45 is involved in cytokinesis and colocalizes with non-muscle myosin in the early Caenorhabditis elegans embryo. J Cell Sci 117:5313–5321
Krone PH, Evans TG, Blechinger SR (2003) Heat shock gene expression and function during zebrafish embryogenesis. Semin Cell Dev Biol 14:267–274
Landsverk ML, Li S, Hutagalung AH, Najafov A, Hoppe T, Barral JM, Epstein HF (2007) The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J Cell Biol 177:205–210
Lee CF, Melkani GC, Bernstein SI (2014) The UNC-45 myosin chaperone: from worms to flies to vertebrates. Int Rev Cell Mol Biol 313:103–144
Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP (1997) Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science 277:383–387
Lord M, Pollard TD (2004) UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J Cell Biol 167:315–325
Lord M, Sladewski TE, Pollard TD (2008) Yeast UCS proteins promote actomyosin interactions and limit myosin turnover in cells. Proc Natl Acad Sci U S A 105:8014–8019
Mandal AK, Lee P, Chen JA et al (2007) Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J Cell Biol 176:319–328
Millson SM, Truman A, Piper PW (2003) Vectors for N- or C-terminal positioning of the yeast Gal4p DNA binding or activator domains. BioTechniques 35:60–64
Millson SH, Truman AW, Wolfram F et al (2004) Investigating the protein-protein interactions of the yeast Hsp90 chaperone system by two-hybrid analysis: potential uses and limitations of this approach. Cell Stress Chaperones 9:359–368
Millson SH, Truman AW, King V, Prodromou C, Pearl LH, Piper PW (2005) A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p). Eukaryot Cell 4:849–860
Mishra M, D’Souza VM, Chang KC, Huang Y, Balasubramanian MK (2005) Hsp90 protein in fission yeast Swo1p and UCS protein Rng3p facilitate myosin II assembly and function. Eukaryot Cell 4:567–576
Ni W, Odunuga OO (2014) UCS proteins: chaperones for myosin and co-chaperones for Hsp90. Subcell Biochem 78:133–152
Niedenthal RK, Riles L, Johnston M, Hegemann JH (1996) Green fluorescent protein as a marker for gene expression and subcellular localisation in budding yeast. Yeast 12:773–786
Price MG, Landsverk ML, Barral JM, Epstein HF (2002) Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J Cell Sci 115:4013–4023
Shi H, Blobel G (2010) UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region. Proc Natl Acad Sci U S A 107:21382–21387
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27
Smythe E, Ayscough KR (2006) Actin regulation in endocytosis. J Cell Sci 119:4589–4598
Sreedhar AS, Kalmar E, Csermely P, Shen YF (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15
Toi H, Fujimura-Kamada K, Irie K, Takai Y, Todo S, Tanaka K (2003) She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol Biol Cell 14:2237–2249
Vaughan CK, Mollapour M, Smith JR et al (2008) Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell 31:886–895
Wendland B, McCaffery JM, Xiao Q, Emr SD (1996) A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol 135:1485–1500
Wesche S, Arnold M, Jansen RP (2003) The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr Biol 13:715–724
Wong KC, Naqvi NI, Iino Y, Yamamoto M, Balasubramanian MK (2000) Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J Cell Sci 113:2421–2432
Acknowledgements
We are indebted to Kathryn Ayscough for her advice concerning the use of FM4-64. S.G-E was supported by a University of Sheffield Krebs Studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gomez-Escalante, S., Piper, P.W. & Millson, S.H. Mutation of the Ser18 phosphorylation site on the sole Saccharomyces cerevisiae UCS protein, She4, can compromise high-temperature survival. Cell Stress and Chaperones 22, 135–141 (2017). https://doi.org/10.1007/s12192-016-0750-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0750-0