Abstract
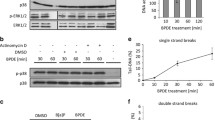
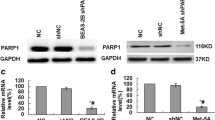
Benzo[a]pyrene (BaP) is a ubiquitously distributed environmental pollutant that induces deoxyribonucleic acid (DNA) damage. The inducible heat shock protein (HspA1A) can function as a molecular chaperone; however, its role in DNA repair remains largely unknown. In the present study, human bronchial epithelial cells (16HBE) stably transfected with plasmids carrying HspA1A gene or shRNAs against HspA1A were treated with BaP. DNA damage levels of the cells were evaluated by comet assay. Results suggest that HspA1A could protect cells against DNA damage and facilitate the decrease of DNA damage levels during the first 2 h of DNA repair. DNA repair capacity (DRC) of Benzo(a)pyrene diol epoxide (BPDE)-DNA adducts was evaluated by host cell reactivation assay in the stable 16HBE cells transfected with luciferase reporter vector PCMVluc pretreated with BPDE. Compared with control cells, cells overexpressing HspA1A showed higher DRC (p < 0.01 at 10 μM BPDE and p < 0.05 at 20 μM BPDE, respectively), while knockdown of HspA1A inhibited DNA repair (p < 0.05 at 10 μM BPDE). Moreover, casein kinase 2 (CK2) was shown to interact with HspA1A by mass spectrometry and co-immunoprecipitation assays. The two proteins were co-localized in the cell nucleus and perinuclear region during DNA repair, and were identified by confocal laser scanning microscope. In addition, cells overexpressing HspA1A showed an increased CK2 activity after BaP treatment compared with control cells (p < 0.01). Our results suggest that HspA1A facilitates DNA repair after BaP treatment. HspA1A also interacts with CK2 and enhances the kinase activities of CK2 during DNA repair.





Similar content being viewed by others
References
Ahmed K, Gerber DA, Cochet C (2002) Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 12:226–230
Ahn B, Kang D, Kim H, Wei Q (2004) Repair of mitomycin C cross-linked DNA in mammalian cells measured by a host cell reactivation assay. Mol Cells 18:249–255
Bases R (2006) Heat shock protein 70 enhanced deoxyribonucleic acid base excision repair in human leukemic cells after ionizing radiation. Cell Stress Chaperones 11:240–249
Beckmann RP, Mizzen LE, Welch WJ (1990) Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 248:850–854
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366
Celotti L, Ferraro P, Furlan D, Zanesi N, Pavanello S (1993) DNA repair in human lymphocytes treated in vitro with (+)-anti- and (+/-)-syn-benzo[a]pyrene diolepoxide. Mutat Res 294:117–126
Chiang HL, Terlecky SR, Plant CP, Dice JF (1989) A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246:382–385
Chirico WJ, Waters MG, Blobel G (1988) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805–810
Filhol O, Cochet C (2009) Protein kinase CK2 in health and disease: cellular functions of protein kinase CK2: a dynamic affair. Cell Mol Life Sci 66:1830–1839
Fleck O, Nielsen O (2004) DNA repair. J Cell Sci 117:515–517
He P, He HZ, Dai J et al (2005) The human plasma proteome: analysis of Chinese serum using shotgun strategy. Proteomics 5:3442–3453
Hightower LE (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66:191–197
Hood JK, Silver PA (2000) Diverse nuclear transport pathways regulate cell proliferation and oncogenesis. Biochim Biophys Acta 1471:M31–M41
Kenny MK, Mendez F, Sandigursky M, Kureekattil RP, Goldman JD, Franklin WA, Bases R (2001) Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J Biol Chem 276:9532–9536
Knudsen NO, Andersen SD, Lutzen A, Nielsen FC, Rasmussen LJ (2009) Nuclear translocation contributes to regulation of DNA excision repair activities. DNA Repair (Amst) 8:682–689
Kotoglou P, Kalaitzakis A, Vezyraki P, Tzavaras T, Michalis LK, Dantzer F, Jung JU, Angelidis C (2009) Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones 14:391–406
Lepock JR, Frey HE, Heynen ML, Senisterra GA, Warters RL (2001) The nuclear matrix is a thermolabile cellular structure. Cell Stress Chaperones 6:136–147
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Litchfield DW (2003) Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369:1–15
Loizou JI, El-Khamisy SF, Zlatanou A et al (2004) The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell 117:17–28
Mendez F, Sandigursky M, Franklin WA, Kenny MK, Kureekattil R, Bases R (2000) Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat Res 153:186–195
Mendez F, Kozin E, Bases R (2003) Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones 8:153–161
Niu P, Liu L, Gong Z, Tan H, Wang F, Yuan J, Feng Y, Wei Q, Tanguay RM, Wu T (2006) Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell Stress Chaperones 11:162–169
Olsen BB, Issinger OG, Guerra B (2010) Regulation of DNA-dependent protein kinase by protein kinase CK2 in human glioblastoma cells. Oncogene 29:6016–6026
Olsen BB, Wang SY, Svenstrup TH, Chen BP, Guerra B (2012) Protein kinase CK2 localizes to sites of DNA double-strand break regulating the cellular response to DNA damage. BMC Mol Biol 13:7
Parsons JL, Dianova II, Finch D, Tait PS, Strom CE, Helleday T, Dianov GL (2010) XRCC1 phosphorylation by CK2 is required for its stability and efficient DNA repair. DNA Repair (Amst) 9:835–841
Shi Y, Brown ED, Walsh CT (1994) Expression of recombinant human casein kinase II and recombinant heat shock protein 90 in Escherichia coli and characterization of their interactions. Proc Natl Acad Sci U S A 91:2767–2771
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Szekely L, Jiang WQ, Pokrovskaja K, Wiman KG, Klein G, Ringertz N (1995) Reversible nucleolar translocation of Epstein–Barr virus-encoded EBNA-5 and hsp70 proteins after exposure to heat shock or cell density congestion. J Gen Virol 76:2423–2432
Wu TC, Tanguay RM, Wu Y et al (1996) Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci 9:370–379
Wu T, Chen S, Xiao C et al (2001) Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones 6:113–120
Xiao C, Chen S, Li J et al (2002) Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones 7:396–402
Yang J, Liu X, Niu P, Zou Y, Gong Z, Yuan J, Wu T (2007) Dynamic changes of XPA, XPC, XPF, XPG and ERCC1 protein expression and their correlations with levels of DNA damage in human bronchial epithelia cells exposed to benzo[a]pyrene. Toxicol Lett 174:10–17
Yang J, Liu X, Niu P, Zou Y, Duan Y (2009) Correlations and co-localizations of Hsp70 with XPA, XPG in human bronchial epithelia cells exposed to benzo[a]pyrene. Toxicology 265:10–14
Yata K, Lloyd J, Maslen S, Bleuyard JY, Skehel M, Smerdon SJ, Esashi F (2012) Plk1 and CK2 act in concert to regulate Rad51 during DNA double strand break repair. Mol Cell 45:371–383
Zou Y, Crowley DJ, Van Houten B (1998) Involvement of molecular chaperonins in nucleotide excision repair. Dnak leads to increased thermal stability of UvrA, catalytic UvrB loading, enhanced repair, and increased UV resistance. J Biol Chem 273:12887–12892
Acknowledgments
This work was supported by the National Outstanding Youth Science Foundation of China [30525031] and the National Natural Science Foundation of China [30872092].
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanying Duan and Suli Huang contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 462 kb)
Rights and permissions
About this article
Cite this article
Duan, Y., Huang, S., Yang, J. et al. HspA1A facilitates DNA repair in human bronchial epithelial cells exposed to Benzo[a]pyrene and interacts with casein kinase 2. Cell Stress and Chaperones 19, 271–279 (2014). https://doi.org/10.1007/s12192-013-0454-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-013-0454-7