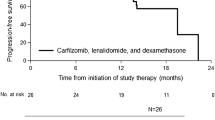
Abstract
We conducted a multicenter, open-label Phase I study of single-agent carfilzomib in Japanese patients with relapsed or refractory multiple myeloma. The primary endpoints were tolerability and safety. Carfilzomib was administrated for 30 min on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. In cycle 1, doses for days 1 and 2 were 20 mg/m2, followed by 45 or 56 mg/m2. Three and four subjects were enrolled in the 20/45 mg/m2 cohort and 20/56 mg/m2 cohort. No dose-limiting toxicity was observed, and the tolerability of carfilzomib was confirmed. Pyrexia, hypertension, nausea and vomiting were considered as noteworthy adverse events (AE) when carfilzomib was administered at high doses. Moreover, pyrexia, blood creatinine increased, and body weight gain were observed as acute dose effects. These findings suggest that addition of dexamethasone is important to alleviate acute dose effect. The overall response rates of the 20/45 mg/m2 and 20/56 mg/m2 cohort were 66.7 % (two out of three) and 50 % (two out of four), respectively. Carfilzomib administrated at up to 20/56 mg/m2 was well tolerated and seemed active in Japanese patients with relapsed or refractory multiple myeloma.
Clinical Trial Registration: JapicCTI-122020.

Similar content being viewed by others
References
Hideshima T, Anderson KC. Novel therapies in MM: from the aspect of preclinical studies. Int J Hematol. 2011;94:344–54.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–74.
Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–91.
Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–25.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Watanabe T, Tobinai K, Matsumoto M, Suzuki K, Sunami K, Ishida T, et al. Phase 1/2 study of carfilzomib in Japanese patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2016;172:745–56.
Yang J, Wang Z, Fang Y, Jiang J, Zhao F, Wong H, et al. Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metab Dispos. 2011;39:1873–82.
Jiang J, Kirk CJ, Muchamuel T, Lee S. The benefits of irreversibility: Infusion administration of carfilzomib results in potent proteasome inhibition and improved safety in animals. Cancer Res. 2011;71:Abstract 2607.
Papadopoulos KP, Siegel DS, Vesole DH, Lee P, Rosen ST, Zojwalla N, et al. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33:732–9.
Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
Willson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12.
Ogawa Y, Tobinai K, Ogura M, Ando K, Tsuchiya T, Kobayashi Y, et al. Phase I and II pharmacokinetic and pharmacodynamic study of the proteasome inhibitor bortezomib in Japanese patients with relapsed or refractory multiple myeloma. Cancer Sci. 2008;99:140–4.
Acknowledgments
We thank all of the patients who participated in this study and their families as well as all investigators, physicians, nurses and clinical research coordinators who helped with this study. We would also like to thank Dr. Hirokazu Murakami (Gunma University Graduate School of Health Science, Maebashi) who was the medical consultant as well as Dr. Yutaka Ariyoshi (Aichi Cancer Center Aichi Hospital, Okazaki), Dr. Chihiro Shimazaki (Japan Community Health care Organisation Kyoto-Kuramaguchi Medical Center, Kyoto), Dr. Masahiro Kizaki (Saitama Medical Center, Saitama Medical University, Saitama), Dr. Takao Katoh (International University of Health and Welfare, Mita Hospital, Tokyo), Dr. Masahiro Endo (Shizuoka Cancer Center, Shizuoka) and Dr. Terufumi Kato (Kanagawa Cardiovascular and Respiratory Center, Yokohama) for their strict review of the clinical data as members of the Efficacy and Safety Monitoring Committee. We also acknowledge the statistical support of Naokazu Gion (Ono Pharmaceutical Co., Ltd., Osaka) and critical review of the manuscript by Sanjay K. Aggarwal, Ying Ou, Sunhee Ro, Michael A. Kelsh, Cristina Carlis (Amgen Inc, Thousand Oaks). This study was supported by Ono Pharmaceutical Co., Ltd. (Osaka).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Iida reports personal fees from Ono Pharmaceuticals Co., Ltd., grants from Ono Pharmaceuticals Co., Ltd., during the conduct of the study; personal fees from Janssen Pharmaceuticals K.K., personal fees from Takeda Pharmaceuticals Co., Ltd., personal fees from Celgene K.K., personal fees from Bristol-Myers Squibb, grants from Chugai Pharmaceuticals, grants from Kyowa Hakko Kirin Co., Ltd., grants from Celgene K.K., grants from Janssen Pharmaceuticals K.K., grants from Eli Lilly Japan K.K., grants from Takeda Pharmaceuticals Co., Ltd., grants from Novartis Pharma K.K., grants from Sanofi K.K., grants from Bristol-Myers Squibb, grants from Bayer Yakuhin Ltd., grants from Toyama Chemical Co., Ltd., grants from Teijin Pharma Ltd., grants from Astellas Pharma Inc., outside the submitted work;. Dr. Tobinai reports grants from Ono Pharmaceutical, during the conduct of the study; grants and personal fees from Eisai, personal fees from HUYA Bioscience, grants and personal fees from Janssen Pharmaceuticals, grants and personal fees from Mundipharma, grants and personal fees from Takeda, personal fees from Zenyaku Kogyo, grants from Abbvie, grants from Celgene, grants from Chugai Pharma, grants from GlaxoSmithKline, grants from Kyowa Hakko Kirin, grants from SERVIER, outside the submitted work. Dr. Taniwaki reports grants from Celgene, grants from Kyowa Hakko Kirin, grants from Chugai Pharma, grants from Janssen Pharma, grants from Novartis, grants from Bristol-Myers Squibb, grants from Pfizer Inc, grants from Takeda Pharma, grants from Asahikasei Pharma, grants from Dainippon Sumitomo Pharma, outside the submitted work. Mr. Shumiya is an employee of Ono Pharmaceutical Co., Ltd. Mr. Nakamura is an employee of Ono Pharmaceutical Co., Ltd. Dr. Chou reports personal fees from Jansen Japan Pharmaceutical Co., Ltd., personal fees from Celgene Japan Pharmaceutical Co., Ltd., personal fees from BMS Japan Pharmaceutical Co., Ltd., personal fees from Takeda Japan Pharmaceutical Co., Ltd., personal fees from Chugai Japan Pharmaceutical Co., Ltd., outside the submitted work; and I received consultation fee from Ono Pharmaceutical Company.
About this article
Cite this article
Iida, S., Tobinai, K., Taniwaki, M. et al. Phase I dose escalation study of high dose carfilzomib monotherapy for Japanese patients with relapsed or refractory multiple myeloma. Int J Hematol 104, 596–604 (2016). https://doi.org/10.1007/s12185-016-2070-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2070-7