Abstract
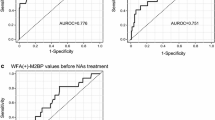
Wisteria floribunda agglutinin-positive human Mac-2-binding protein (WFA+-M2BP) was developed recently as a predictive marker of progression to liver fibrosis and hepatocellular carcinoma (HCC) in patients seropositive for hepatitis C virus (HCV). We retrospectively analyzed 16 HCV-seropositive patients who received systemic chemotherapy for hematologic malignancies to evaluate the usefulness of WFA+-M2BP for predicting HCV-related complications. These were defined as the onset of significant liver damage (LD) with increased HCV RNA levels, leading to interrupted or discontinued chemotherapy or the occurrence of HCC after chemotherapy. Baseline WFA+-M2BP levels were determined using preserved serum samples. The median level of WFA+-M2BP was 1.59 [cutoff index (C.O.I.) value range 0.38–6.66]. With a median follow-up of 623 days (range 120–2404), LD and HCC were observed in three and two patients, respectively. Detectable HCV RNA and WFA+-M2BP ≥2.0 C.O.I. at baseline were identified as risk factors for these HCV-related complications (P = 0.034 and P = 0.005, respectively). The sensitivity, specificity, and positive and negative predictive values of the WFA+-M2BP level (cutoff point: 2.0 C.O.I.) for the occurrence of HCV-related complications were 100.0, 81.8, 71.4, and 100.0 %, respectively. WFA+-M2BP may be a useful marker for the prediction of HCV-related complications in HCV-seropositive patients following systemic chemotherapy.



Similar content being viewed by others
Abbreviations
- WFA+-M2BP:
-
Wisteria floribunda agglutinin-positive human Mac-2-binding protein
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HBV:
-
Hepatitis B virus
- LD:
-
Liver damage
- LC:
-
Liver cirrhosis
- C.O.I.:
-
Cutoff index
- ALT:
-
Alanine transaminase
- LF:
-
Liver failure
- PT:
-
Prothrombin time
- ROC:
-
Receiver operating characteristic
- IFN:
-
Interferon
- DAA:
-
Direct-acting antiviral
References
Lauer GM, Walker BD. Hepatitis C virus infection. New England J Med. 2001;345:41–52.
Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. New England J Med. 1995;332:1463–6.
De Mitri MS, Poussin K, Baccarini P, Pontisso P, D’Errico A, Simon N, et al. HCV-associated liver cancer without cirrhosis. Lancet. 1995;345:413–5.
Pioltelli P, Zehender G, Monti G, Monteverde A, Galli M. HCV and non-Hodgkin lymphoma. Lancet. 1996;347:624–5.
Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125:1723–32.
Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745–52.
Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, et al. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158–65.
Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J Clin Oncol Off J American Soc Clin Oncol. 2005;23:468–73.
Besson C, Canioni D, Lepage E, Pol S, Morel P, Lederlin P, et al. Characteristics and outcome of diffuse large B-cell lymphoma in hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe d’Etude des Lymphomes de l’Adulte programs. J Clin Oncol Off J American Soc Clin Oncol. 2006;24:953–60.
Visco C, Arcaini L, Brusamolino E, Burcheri S, Ambrosetti A, Merli M, et al. Distinctive natural history in hepatitis C virus positive diffuse large B-cell lymphoma: analysis of 156 patients from northern Italy. Ann Oncol Off J Euro Soc Med Oncol/ESMO. 2006;17:1434–40.
Arcaini L, Merli M, Passamonti F, Bruno R, Brusamolino E, Sacchi P, et al. Impact of treatment-related liver toxicity on the outcome of HCV-positive non-Hodgkin’s lymphomas. Am J Hematol. 2010;85:46–50.
Ennishi D, Maeda Y, Niitsu N, Kojima M, Izutsu K, Takizawa J, et al. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood. 2010;116:5119–25.
Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nature Rev Clin Oncol. 2012;9:156–66.
Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, et al. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177–85.
Watanabe T, Tanaka Y. Reactivation of hepatitis viruses following immunomodulating systemic chemotherapy. Hepatol Res Off J Japan Soc Hepatol. 2013;43:113–21.
Merli M, Visco C, Spina M, Luminari S, Ferretti VV, Gotti M, et al. Outcome prediction of diffuse large B-cell lymphomas associated with hepatitis C virus infection: a study on behalf of the Fondazione Italiana Linfomi. Haematologica. 2014;99:489–96.
Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065.
Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76–84.
Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50:776–84.
Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563–70.
Sasaki R, Yamasaki K, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis c after sustained virological response. PLoS ONE. 2015;10:e0129053.
Tamaki N, Kurosaki M, Kuno A, Korenaga M, Togayachi A, Gotoh M, et al. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol Res Off J Japan Soc Hepatol. 2015;45:E82–8.
Fujiyoshi M, Kuno A, Gotoh M, Fukai M, Yokoo H, Kamachi H, et al. Clinicopathological characteristics and diagnostic performance of Wisteria floribunda agglutinin positive Mac-2-binding protein as a preoperative serum marker of liver fibrosis in hepatocellular carcinoma. J Gastroenterol. 2015;50:1134–44.
Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7.
Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. New England J Med. 2012;366:216–24.
Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. New England J Med. 2013;368:34–44.
Acknowledgments
We thank Ms. Chiori Fukuyama and Dr. Shintaro Ogawa (Nagoya City University Graduate School of Medical Sciences, Nagoya) for storing and measuring the samples used in this study. This study was supported in part by the Research Program on Hepatitis from Japan Agency for Medical Research and development, AMED (Y.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yasuhito Tanaka: Research funding and honoraria from Sysmex Corporation, Bristol-Myers Squibb Co., Chugai Pharmaceutical Co. Ltd. Masaki Ri: Research funding from Celgene K.K. Takashi Ishida: Research funding from Kyowa Hakko Kirin Co., Ltd., Bayer Pharma AG, and Celgene K.K., and honoraria from Kyowa Hakko Kirin Co., Ltd. Shinsuke Iida: Research funding from Bristol-Myers Squibb Co., Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Celgene K.K., Kyowa Hakko Kirin Co. Ltd., Ono Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Nippon Kayaku Co. Ltd., and honoraria from Celgene K.K., Janssen pharmaceutical K.K. and Ono Pharmaceutical Co. Ltd.
About this article
Cite this article
Totani, H., Kusumoto, S., Tanaka, Y. et al. The value of serum Wisteria floribunda agglutinin-positive human Mac-2-binding protein as a predictive marker for hepatitis C virus-related complications after systemic chemotherapy. Int J Hematol 104, 384–391 (2016). https://doi.org/10.1007/s12185-016-2033-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2033-z