Abstract
Peripheral T cell lymphoma (PTCL) represents a heterogeneous group of mature T and natural killer cell-derived neoplasms, comprising approximately 10 % of non-Hodgkin lymphoma. Although at least 20 distinct histologic subtypes of PTCL have been identified, the historical treatment approach has been uniform application of anthracycline-based combination chemotherapy, resulting in significantly inferior outcomes compared to B-cell non-Hodgkin lymphoma. Because of the generally poor outcomes with conventional chemotherapy, PTCL represents an unmet medical need, therefore providing the opportunity to evaluate novel agents. Herein, we will review the evolving treatment strategies for PTCL, discuss how different treatment approaches impact the underlying biology of PTCL, and speculate on future targets for therapeutic intervention. We conclude that future efforts to develop effective therapies for PTCL will benefit from a biomarker-driven strategy rather than histologic classification.
Similar content being viewed by others
Introduction
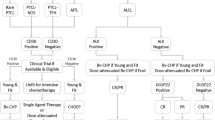
Peripheral T cell lymphoma (PTCL) is a basket term used to describe a diverse group of mature T and natural killer (NK) cell malignancies, comprising at least 20 distinct subtypes [1]. PTCL represents approximately 10 % of all non-Hodgkin lymphoma (NHL). The International PTCL Study represents the largest systematic effort to examine the pathologic and clinical characteristics of PTCL, examining over 1300 cases compiled from 22 centers worldwide [2]. The study found that the most common types of PTCL are peripheral T cell lymphoma, not otherwise specified (PTCL-NOS) at 25.9 % and angioimmunoblastic T cell lymphoma (AITL) at 18.5 %. The third most common form of PTCL is anaplastic large cell lymphoma (ALCL), which can be subdivided by expression of the anaplastic lymphoma kinase (ALK) into ALK+ versus ALK− ALCL, representing 6.6 and 5.5 % of PTCL, respectively. The overall frequencies of the various PTCL entities are depicted graphically in Fig. 1.
Relative frequencies of PTCL subtypes according to the International PTCL Project adapted from [2]. PTCL-NOS peripheral T cell lymphoma, not otherwise specified, AITL angioimmunoblastic T cell lymphoma, ALCL anaplastic large cell lymphoma, NKTCL natural killer/T cell lymphoma, ATLL adult T cell leukemia/lymphoma, ETTL enteropathy-type T cell lymphoma, PC-ALCL primary cutaneous anaplastic large cell lymphoma, HSTCL hepatosplenic T cell lymphoma, SPLTCL subcutaneous panniculitis-like T cell lymphoma
The relative distribution of PTCL subtypes varies by geographic region. In North America, ALK+ ALCL is more common, representing approximately 16 % of PTCL. AITL and enteropathy-type T-cell lymphoma (ETTL) are overrepresented in Europe where they comprise 28.7 and 9.1 % of PTCL, respectively. In Asia, natural killer/T cell lymphoma (NKTCL) and adult T cell leukemia/lymphoma (ATLL) account for 22.4 and 25 % of PTCL, respectively. The reasons for the different geographic distributions are only partially understood: ATLL is caused by infection with HTLV-1, a retrovirus with higher prevalence in Japan (and the Caribbean); ETTL may have a higher frequency in Northern Europe because it is associated with gluten-sensitive enteropathy, a disease prevalent in that region. Table 1 highlights differences in distribution of PTCL subtypes by geographic region.
The relative rarity and phenotypic heterogeneity of PTCL make it difficult to study in a rigorous manner, much less conduct large clinical trials [3, 4]. Of the estimated 70,000 cases of NHL diagnosed in the US in 2013, approximately 7000 are PTCL (www.seer.cancer.gov). Therefore, even the most common subtypes of PTCL account for only 1000–2000 cases per year in the US, while the more rare subtypes number <100 cases per year. This review will focus primarily on treatment of the most common subtypes of PTCL (PTCL-NOS, AITL, ALCL, ATLL), for which there are more data available.
Current treatment approaches
Newly diagnosed patients
Most patients with PTCL present with advanced stage disease, thus necessitating systemic therapy [2]. Moreover, many patients with PTCL present with cytopenias and/or a poor performance status, making it challenging to manage the toxicities of systemic chemotherapy [5, 6]. Treatment regimens were and still are selected based on the underlying histology, but this treatment paradigm did little to advance therapeutic strategies or improve patient outcomes.
Historically, PTCL was considered a subtype of non-Hodgkin lymphoma, thus the treatment regimens mirrored those of B-cell NHL. The most commonly used initial regimens have been anthracycline-based combination chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), despite recognition that patients with PTCL have significantly inferior outcomes in response to these regimens compared to patients with B-cell NHL [7]. The notable exceptions are ALK+ ALCL and nasal-type NKTCL, which have relatively favorable outcomes to chemotherapy or radiation ± chemotherapy, respectively [2].
In the International PTCL Study, greater than 85 % of patients with the most common subtypes of PTCL (PTCL-NOS, AITL, ALCL, ATLL) received up-front therapy with an anthracycline-based combination chemotherapy regimen [2]. There were distinct 5-year overall survival (OS) depending on histologic subtype. PTCL-NOS and AITL each had 5-year OS of 32 %. ATLL did most poorly with a 5-year OS of 14 %, while ALK-positive ALCL did best with a 5-year OS of 70 %. Five-year failure-free survival (FFS) was 20 % for PTCL-NOS, 18 % for AITL, and 12 % for ATLL. Here again, ALCL exhibited superior outcomes with 5-year FFS of 60 % for ALK + ALCL and 36 % for ALK− ALCL. In this large dataset, there was no clear value to include an anthracycline as a component of the treatment regimen. Note that the superior outcomes for patients with ALCL are important to keep in mind when assessing reported outcomes for different therapeutic approaches, as trials tend to vary greatly in the relative proportions of ALCL patients.
A recent large meta-analysis examined all available published data on over 2800 patients with PTCL. Up-front treatment with anthracycline-based combination chemotherapy resulted in a complete response (CR) rate of 52 % and 5-year OS of 35 % for PTCL (excluding ALCL) [8]. Outcomes with anthracycline-based combination chemotherapy varied amongst different PTCL subtypes, ranging from a CR rate of 36 % and 5-year OS of 20 % for enteropathy-type T cell lymphoma (ETTL) to a CR rate of 66 % and 5-year OS of 57 % for anaplastic large cell lymphoma (ALCL). The authors acknowledge that these results might overestimate real-life outcomes, as the meta-analysis population was comprised primarily of a selected group of patients that had been treated in phase II trials.
Several prospective studies offer further insight into the response rates and durability of responses to CHOP chemotherapy in the frontline setting. In a study by Reimer and colleagues, 83 patients with newly diagnosed PTCL received CHOP chemotherapy as induction treatment, resulting in a high objective response rate (ORR) of 79 % and CR rate of 39 % [9]. Nonetheless, even though 66 % of these patients went on to receive consolidative high-dose therapy with autologous stem cell rescue (HDT/ASCR), the 3-year PFS was only 36 % [9]. A retrospective series from British Columbia analyzed the outcomes of 117 patients with PTCL-NOS treated with up-front CHOP. Despite the ORR of 84 % and CR rate of 64 %, the durability of response was poor with a 5-year PFS of 29 % [10]. Several other studies reported similar outcomes [11, 12].
Given the disappointing outcomes for PTCL, numerous attempts have been made to improve upon CHOP chemotherapy. The German High-Grade NHL Study Group compiled data from 7 different clinical trials to investigate whether the addition of etoposide to CHOP (CHOEP) was associated with improved outcomes for patients with PTCL compared to CHOP alone [13]. For patients <60 years of age with a normal LDH, the addition of etoposide to CHOP was associated with a significant improvement in EFS. Of note, 56 % of patients in this trial had ALCL, which, as mentioned previously, tends to do more favorably than other subtypes of PTCL. For ALK+ ALCL patients treated with CHOEP the 3-year EFS was 91 versus 57 % for those treated with CHOP (p = 0.012). Excluding ALK+ ALCL patients, the results demonstrated a non-significant trend favoring CHOEP with a 3-year PFS of 61 versus 48 % for CHOP alone (p = 0.57). Based on these findings CHOEP has become standard of care for patients <60 years of age with ALK+ ALCL (NCCN guidelines).
More intensive anthracycline-containing chemotherapy regimens such as VIP-rABVD (etoposide, ifosfamide, cisplatin, doxorubicin, bleomycin, vinblastine, dacarbazine) have not demonstrated clear improvements over CHOP [12]. One group hypothesized that the poor response of PTCL to CHOP-like chemotherapy might be due to overexpression of the multidrug resistance gene 1/P-glycoprotein (MDR1/P-gp), which would promote efflux of chemotherapy from the cell. Thus, a novel non-anthracycline-containing regimen called PEGS (cisplatin, etoposide, gemcitabine, methylprednisolone) was developed and tested prospectively in 44 patients with PTCL [14]. The PEGS regimen, however, appeared to be inferior to CHOP with an ORR of 39 %, CR rate of 24 %, and 2-year PFS of 12 %. Attempts to add novel agents to the CHOP backbone will be discussed later.
Consolidation in first remission
Consolidation in first remission with high-dose therapy and autologous stem cell rescue (HDT/ASCR) remains an area of controversy for PTCL. Given the lack of prospective randomized data, it remains difficult to determine whether the patients that do well after HDT/ASCR are the same group that would have done well with chemotherapy alone. Nonetheless, NCCN and other guidelines recommend consolidation with HDT/ASCR in first remission (excluding ALK+ ALCL).
A prospective German trial treated 83 patients with CHOP induction followed by HDT/ASCR [9]. A significant portion of patients (34 %) experienced progressive disease during induction and could not go on to HDT/ASCR. In an intention-to-treat analysis, CHOP followed by HDT/ASCR resulted in a 3-year PFS of 36 %, which may represent a marginal improvement over CHOP alone.
Etoposide-containing induction regimens such as CHOEP (n = 160) or sequential CHOP then ICE (ifosfamide, carboplatin, etoposide) (n = 65) followed by HDT/ASCR yielded somewhat better results with 5-year and 4-year PFS of 44 %, respectively [11, 15]. Subgroup analyses demonstrated that patients with ALK− ALCL had particularly good outcomes with this approach with 5-year PFS of 61 % [15]. Additional factors predicting for benefit include low-risk disease by IPI or PIT classification and a complete response prior to transplant, whereas patients with high-risk disease by IPI and less than a CR on interim PET do not appear to benefit from HDT/ASCR in first remission [9, 11].
In summary, consolidation of first remission with HDT/ASCR likely provides a modest benefit in terms of PFS and OS for patients with PTCL. However, this treatment strategy applies only to younger and fitter patients without significant comorbidities. Moreover, this approach does not apply to the one-third of patients with disease progression during induction.
Relapsed/refractory disease
In contrast to consolidation with HDT/ASCR in the up-front setting, there does not seem to be much benefit to HDT/ASCR for patients with relapsed/refractory PTCL [16]. The goals of treatment for patients with relapsed/refractory disease depend on whether or not allogeneic stem cell transplantation (alloSCT) is an option [17]. If the patient is a candidate for alloSCT and a suitable match is available, then the priority is to treat with a second-line regimen that has a high response rate, so that the patient can go into the transplant with a CR or at least a good partial response (PR). Typical second-line regimens include ICE, DHAP (dexamethasone, cytarabine, cisplatin), ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin), or GCD (gemcitabine, carboplatin, dexamethasone). In the highly selected group of patients that undergo allogeneic transplant for relapsed/refractory PTCL, there is evidence for a graft-versus-lymphoma effect, and long-term survival rates can be as high as 50 % [18–20].
For the majority of patients with relapsed/refractory PTCL, allogeneic stem cell transplantation is not an option either due to advanced age, comorbidities, lack of a suitable stem cell donor, or inability to obtain an adequate response. In this setting, the priority is disease control for as long as possible with as few treatment-related toxicities as possible [17]. Ideally, disease control can be achieved with prolonged and sequential treatments with single-agents therapies, many of which are described in the remainder of this review. The ultimate goal for the treatment of PTCL will be to move the most effective and least toxic single agents or combinations of agents to the up-front setting to increase the rate of cure.
Pralatrexate, romidepsin, and brentuximab vedotin are the only three FDA-approved agents for the treatment of relapsed or refractory PTCL. A number of other drugs with FDA approval for other indications are being studied in PTCL, including bendamustine, denileukin diftitox, vorinostat, 5-azacitidine, bortezomib, lenalidomide, bevacizumab, crizotinib, and imatinib. In addition, there are numerous investigational agents in various stages of development. The clinical activities of the various agents approved or under investigation for the treatment of PTCL are summarized in Fig. 2 and Table 2, and will be described in detail below.
Activity of therapeutic agents for PTCL. Chart includes agents with phase II clinical trial data testing drug as a single agent in relapsed or refractory PTCL and/or specific subsets of PTCL, with exceptions indicated in parentheses. CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) in newly diagnosed, untreated PTCL patients is included for comparison. N indicates number of patients treated, CR complete response, PR partial response. References: [24, 27, 33, 36, 47, 52, 70, 74, 85, 93, 97]
Chemotherapeutic agents
Pralatrexate
Pralatrexate functions as a potent inhibitor of folate metabolism that preferentially accumulates in cancer cells overexpressing the reduced folate carrier-1 (RFC-1) [21]. Initial studies in patients with non-Hodgkin lymphoma suggested an enhanced activity in T cell lymphoma [22, 23]. In the pivotal phase II PROPEL trial, 111 patients with relapsed/refractory PTCL were treated with pralatrexate weekly for 6 weeks on, 1 week off of every 7-week cycle [24]. The treatment resulted in an ORR of 29 %, CR rate of 11 %, disease control rate (DCR) of 49 %, median duration of response (DoR) of 10.1 months, median PFS of 3.5 months, and median OS of 14.5 months. The primary grade 3/4 toxicities were thrombocytopenia (33 %) and mucositis (22 %), the latter of which could be ameliorated by pretreating patients with vitamin B12 and folate. There are several ongoing clinical trials incorporating pralatrexate into up-front treatment strategies. In one trial, patients with newly diagnosed PTCL receive CHOP or CHOEP induction therapy followed by randomization to pralatrexate maintenance (NCT01420679). A second trial treats newly diagnosed PTCL patients with CEOP alternating with pralatrexate followed by HDT/ASCR (NCT01336933).
Bendamustine
Bendamustine possesses a bifunctional structure with characteristics of both an alkylator and a purine analog, although functionally it appears to act primarily as an alkylating agent [25]. Bendamustine was originally developed in East Germany, which delayed its clinical adaptation by the rest of the world. Bendamustine in combination with rituximab has demonstrated remarkable efficacy for a variety of indolent B-cell NHLs [26]. The BENTLY trial tested bendamustine as a single agent in 60 patients with relapsed/refractory PTCL. Bendamustine 120 mg/m2 IV was administered on days 1 and 2 of every 3-week cycle. The study demonstrated an ORR of 50 %, a CR rate of 28 %, median DoR of 3.5 months, median PFS of 3.6 months, and median OS of 6.2 months [27]. The primary grade 3/4 toxicities observed were neutropenia (30 %), thrombocytopenia (24 %), and infection (20 %). Based on these results, single-agent bendamustine represents an effective means of achieving remission, however, the poor durability of responses suggests that this agent might be better utilized in combination with other agents.
Monoclonal antibodies
The monoclonal antibody rituximab dramatically improved outcomes for patients with aggressive B-cell NHL when added to CHOP chemotherapy [28, 29]. A monoclonal antibody with comparable efficacy to rituximab has not yet been developed for the treatment of PTCL. There are a number of cell surface antigens on T cells that have been targeted for therapeutic intervention, including CD2, CD4, CD30, CD52, and CCR4, as will be discussed below.
Mogamulizumab
Adult T cell leukemia/lymphoma (ATLL) represents a PTCL subtype with one of the worst outcomes in response to CHOP-like chemotherapy, with a 5-year OS of 14 % [2]. ATLL cells express high levels of the chemokine receptor CCR4 [30], which correlates with skin involvement and inferior outcomes [31]. Mogamulizumab, also known as KW-0761, is CCR4-directed monoclonal antibody, engineered with a defucosylated Fc region that enhances antibody-dependent cellular cytotoxicity [32].
A phase II study of mogamulizumab in 28 patients with relapsed, aggressive ATLL demonstrated an ORR of 50 % (13/26 evaluable), including 8 CRs [33]. The median PFS was 5.2 months and the median OS 13.7 months. Interestingly, the drug was most effective for disease localized to the blood. Adverse events included a high rate of infusion reactions (89 %) and skin rash (63 %). The drug was subsequently approved in Japan for the treatment of relapsed/refractory ATLL. There is an ongoing randomized phase III trial testing a multi-agent chemotherapy regimen with or without mogamulizumab for patients with untreated ATLL (NCT01173887). Additional studies with mogamulizumab have demonstrated efficacy in mycosis fungoides and Sezary syndrome [34]. Importantly, up to 30 % of non-ATLL peripheral T cell lymphomas express CCR4, thus making mogamulizumab a potential biomarker-driven therapeutic strategy for PTCL in general [35].
Zanolimumab
The humanized monoclonal antibody zanolimumab targets the T cell co-receptor CD4, a surface protein expressed by normal helper T cells and a significant fraction of PTCL. In a phase II trial, 21 patients with relapsed/refractory PTCL received zanolimumab, resulting in an ORR of 24 %, including 3 CRs [36]. The drug was well tolerated without significant toxicities. There was a trial investigating the combination of zanolimumab plus CHOP for patients with newly diagnosed CD4+ PTCL, but the current status of this trial is unclear (NCT00893516).
Siplizumab
CD2 is a cell surface adhesion molecule highly expressed by normal T lymphocytes and NK cells, as well as their malignant counterparts. Siplizumab is a monoclonal antibody directed against CD2 that has primarily been utilized in the solid organ transplant setting to prevent graft rejection. Despite promising initial results, at least one trial of siplizumab in patients with T cell lymphoma was halted due to an increased rate (~14 %) of EBV-induced B-cell lymphomas, a serious side effect that might limit its clinical utility [37]. Nonetheless, a phase I trial of siplizumab plus dose-adjusted-EPOCH-R is underway for patients with PTCL, with rituximab included in the regimen as a potential means of preventing EBV-induced B-cell lymphomas (NCT01445535).
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody directed at CD52, a cell surface protein expressed by normal B and T lymphocytes, monocytes, and dendritic cells, as well as a significant fraction of PTCL [38, 39]. A phase I trial of single-agent alemtuzumab in 14 patients with relapsed/refractory PTCL demonstrated an ORR of 36 %, including 3 CRs [40]. Combination of alemtuzumab with different schedules of CHOP chemotherapy in the up-front setting demonstrated high ORR ranging from 75 to 90 % and CR rates ranging from 60 to 71 % [41–43]. Despite the impressive up-front responses, over half of patients relapsed within 2 years with PFS/EFS ranging from 27 to 48 %. Importantly, alemtuzumab in combination with chemotherapy was profoundly immunosuppressive with relatively high rates of severe toxicity, including grade 4 infections (17 %), EBV-related B-cell lymphomas (15 %), and treatment-related mortality (10–20 %). Thus, this approach appears to result in excess toxicity without clear benefit. There was an ongoing phase III trial randomizing patients with newly diagnosed PTCL to CHOP or CHOP plus alemtuzumab (NCT00725231), however, the clinical development of alemtuzumab will likely cease, as the parent company recently removed it from the marketplace for financial reasons.
Drug conjugates
Antibody drug conjugates (ADCs) consist of a monoclonal antibody fused to a chemotherapeutic agent via a chemical linker. In theory, ADCs allow for precise delivery of a potent chemotherapeutic agent to a cell expressing a specific surface protein, with little to no off-target toxicity. The development of an effective ADC involves identification of a surface protein that is selectively expressed on malignant cells as well as a linker molecule that is cleaved only upon entry into the target cell. Recent advances in linker chemistry have resulted in the development of several clinically important ADCs. Cytokine toxin fusion molecules are conceptually related to ADCs, but use a cytokine instead of an antibody to mediate the precision delivery of toxin to target cells expressing the receptor for the cytokine.
Brentuximab vedotin
CD30 is a transmembrane receptor of the TNF superfamily that is expressed by several subsets of activated lymphocytes, as well as their malignant counterparts, including all ALCL, a significant fraction of PTCL-NOS, as well as classical Hodgkin lymphoma. As CD30 exhibits limited expression on non-lymphoid cells, it presents an attractive target for potential therapeutic intervention. Initial attempts at targeting the molecule with a naked monoclonal antibody, SGN-30, demonstrated modest clinical activity [44, 45].
Subsequent efforts resulted in the generation of brentuximab vedotin, also known as SGN-35, an anti-CD30 monoclonal antibody fused via a protease-cleavable linker to the potent anti-tubulin agent monomethyl auristatin E (MMAE). Upon binding to CD30 on the surface of the cell, the complex is internalized and trafficked to the lysosomal compartments where the linker undergoes cleavage, and the MMAE toxin is released into the cell. MMAE poisons the microtubule apparatus, resulting in cell cycle arrest and apoptosis.
Brentuximab vedotin was initially developed in relapsed/refractory Hodgkin lymphoma and ALCL, where it demonstrated remarkable efficacy [46]. Brentuximab vedotin was subsequently FDA approved based on a phase II study of 58 patients with relapsed/refractory systemic ALCL demonstrating an impressive ORR of 86 %, CR rate of 57 %, and median duration of response of 13.2 months [47]. The primary adverse events were peripheral sensory neuropathy (41 % any grade, 12 % grade ≥3) and neutropenia (21 % grade ≥3).
Brentuximab vedotin is currently being tested in patients with relapsed/refractory CD30+ PTCL subtypes other than ALCL. Preliminary analysis of the first 29 patients enrolled demonstrated an ORR of 36 %, with a higher ORR of 50 % in the AITL subset, including 4 complete responses [48]. Interestingly, there did not appear to be any correlation between response and level of CD30 expression.
Not surprisingly, there are efforts to move brentuximab vedotin up front in combination with chemotherapy for patients with newly diagnosed CD30+ PTCL. Preliminary results from a phase I trial of 26 patients treated with brentuximab vedotin plus CHP chemotherapy demonstrated an ORR of 100 % and CR rate of 88 % [49]. Peripheral sensory neuropathy occurred in 48 % and febrile neutropenia in 19 % of patients. A double-blind, randomized, multicenter, phase III study known as ECHELON-2 is underway to compare the efficacy and safety of brentuximab vedotin plus CHP to CHOP alone in patients with previously untreated CD30+ PTCL (NCT01777152).
Denileukin diftitox
Interleukin-2 (IL-2) functions as a critical growth factor for T cell proliferation and survival. Denileukin diftitox is a chimeric molecule composed of human IL-2 fused to diphtheria toxin. Denileukin diftitox binds to the IL-2 receptor on normal and malignant T cells, resulting in endocytosis and subsequent apoptosis of the target cell. The drug underwent initial clinical development in cutaneous T cell lymphoma where it received FDA approval [50, 51]. It is currently in late stages of development for PTCL. As a single agent for PTCL, denileukin diftitox exhibited a 48 % objective response rate with a median PFS of 6 months [52]. Interestingly, expression of the high-affinity IL-2 receptor, CD25, did not predict response. The CONCEPT study was a phase II trial testing the addition of denileukin diftitox (DD) to CHOP chemotherapy in 49 patients with newly diagnosed PTCL [53]. The DD-CHOP regimen resulted an ORR of 65 % with a median PFS of 12 months.
LMB-2
The high-affinity IL-2 receptor, CD25, can also be targeted by LMB-2, a recombinant immunotoxin comprised of the variable domains of an anti-CD25 monoclonal antibody fused to a Pseudomonas endotoxin. A phase I trial of LMB-2 in 35 patients with various hematologic malignancies demonstrated profound but transient responses in several patients with ATLL [54]. This formed the rationale for an ongoing phase II trial combining LMB-2 with fludarabine and cyclophosphamide in patients with ATLL (NCT00924170).
Epigenetic-modifying agents
Gene expression undergoes dynamic regulation via post-translational modifications of histones. In general, histone acetylation results in activation of gene expression, whereas histone deacetylation leads to repression of gene expression. Histone deacetylases (HDACs) represent a class of enzymes that catalyze deacetylation of histones, as well as numerous non-histone proteins. Inhibition of HDACs in malignant cells can reactivate expression of aberrantly silenced genes. As a class, HDAC inhibitors have pleiotropic effects on malignant cells including altered gene expression, cell cycle arrest, and activation of apoptosis [55]. Multiple histone deacetylase inhibitors have been investigated as therapies for PTCL, with several exhibiting striking activity. Recent advances regarding the biology of AITL and PTCL-NOS have shed light on why epigenetic-modifying agents might be so effective in these diseases.
Research in basic immunology identified a novel class of T cells known as follicular helper T cells (TFH), a specialized T cell subset that resides within the germinal center and provides T cell help to germinal center B cells [56]. Subsequently, the follicular helper T cell was identified as the probable cell-of-origin for AITL [57, 58]. Meanwhile, gene expression profiling studies confirmed the long-standing notion that PTCL-NOS was comprised of heterogeneous entities [59, 60], with a significant fraction possessing a gene signature consistent with a TFH cell-of-origin [58].
The notion of a common cell-of-origin for AITL and TFH-like PTCL-NOS was strengthened by identification of a common gene mutation pattern in these two PTCL subtypes. Deep sequencing approaches demonstrated that AITL and TFH-like PTCL-NOS share a mutation pattern resembling that seen in myeloid malignancies, with recurrent mutations in TET2, DNMT3A, and IDH2 [61–63]. TET2 and DNMT3A mutations result in hypermethylation of DNA CpG islands, which in turn promote repressive modifications of histone proteins via recruitment of histone deacetylase containing complexes [64]. Mutant IDH2 produces the “oncometabolite” 2-hydroxyglutarate (2HG), which functions as an inhibitor of a variety of chromatin-modifying enzymes, including TET2. More recent genomic analyses of AITL have also detected recurrent loss of function mutations in the histone acetyltransferases EP300 and CREBBP [65]. The net effect of observed gene mutations in AITL and TFH-like PTCL-NOS is a compacted chromatin structure that represses expression of tumor suppressor genes and blocks activation of genes involved in differentiation [66–69]. Thus, therapeutic agents that shift the balance to histone acetylation and DNA demethylation have a strong biologic rationale in these diseases.
Romidepsin
Romidepsin, also known as depsipeptide, is a histone deacetylase inhibitor with FDA approval for treatment of relapsed/refractory PTCL. In the pivotal phase II trial, romidepsin 14 mg/m2 was administered intravenously to 131 patients with relapsed/refractory PTCL on days 1, 8, and 15 of every 28-day cycle. The investigators reported an ORR of 25 %, CR rate of 15 %, and median PFS of 4 months [70]. The most remarkable finding was the long median duration of response measuring 17 months. The most common grade ≥3 toxicities were thrombocytopenia (24 %), neutropenia (20 %), and infections (19 %). Other notable toxicities included nausea (60 % any grade) or asthenia (40 % any grade). Another phase II trial of romidepsin in 47 patients with relapsed/refractory PTCL demonstrated an ORR of 38 % [71]. Romidepsin additionally received FDA approval for the treatment of relapsed cutaneous T cell lymphoma [72, 73]. Ongoing clinical trials for patients with PTCL are testing romidepsin in combination with a variety of agents/regimens, including CHOP (NCT01280526), ICE (NCT01590732), GDP (gemcitabine, dexamethasone, cisplatin) (NCT01846390), pralatrexate (NCT01947140), gemcitabine (NCT01822886), bortezomib (NCT00963274), lenalidomide (NCT01742793), 5-azacitidine (NCT01998035), and alisertib (NCT01897012).
Belinostat
Like romidepsin, belinostat functions as a pan-HDAC inhibitor. The pivotal phase II BELIEF trial was recently completed in which 120 patients with relapsed/refractory PTCL were treated with intravenous belinostat on days 1–5 of every 3-week cycle. The results have been presented in preliminary form with a reported ORR of 26 % and CR rate of 10 % [74]. The median time to response was 5.6 weeks, median DoR was 8.3 months, and longest DoR was 29.4 months. The drug was well tolerated with primarily hematologic grade 3/4 toxicities, including thrombocytopenia (13 %), neutropenia (13 %), and anemia (10 %). Interestingly, there was an apparent preferential activity of belinostat in AITL with an ORR 46 %, consistent with the high frequency of mutations in chromatin-modifying factors in this PTCL subtype [61–63, 65]. Future efforts to correlate response to mutation profile will provide important insights into the mechanism of action of HDACs in PTCL.
Vorinostat
Vorinostat, also known as suberoylanilide hydroxamic acid (SAHA), is an orally available HDAC inhibitor that received FDA approval in 2006 for the treatment of relapsed/refractory CTCL [75]. There are ongoing trials testing vorinostat in patients with PTCL, either alone (NCT00007345) or in combination with various agents, including CHOP (NCT00787527), ICE (NCT00601718), niacinamide plus etoposide (NCT00691210), bortezomib (NCT00810576), 5-azacitidine (NCT00336063), and alisertib (NCT01567709).
Panobinostat
Panobinostat is a potent, orally available HDAC inhibitor that has primarily been under investigation in CTCL [76, 77]. Panobinostat is still in the early stages of clinical development for the treatment of PTCL (NCT01261247). One ongoing clinical trial is testing the combination of panobinostat and bortezomib in patients with relapsed/refractory PTCL (NCT00901147). A case report described a complete response in one patient with PTCL-NOS from that trial [78].
5-Azacitidine
5-Azacitadine is a DNA hypomethylating agent that has FDA approval for the treatment of myelodysplastic syndrome. The combination of a DNA hypomethylating agent with a HDAC inhibitor exhibited striking synergy in preclinical models of T cell lymphoma [79]. Ongoing clinical trials are testing the combination of 5-azacitidine and romidepsin (NCT01998035) or vorinostat (NCT00336063) for patients with relapsed/refractory lymphomas, including PTCL.
Proteasome inhibitors
Proteasomes maintain cellular protein homeostasis via degradation of proteins tagged for elimination and clearance of misfolded proteins [80]. The NF-κB signaling pathway represents a critical mediator of lymphocyte survival. A variety of oncogenic insults result in constitutively activated NF-κB in lymphomas, including many subtypes of PTCL [81]. Activation of NF-κB signaling depends on an intact proteasome system to promote the degradation of the inhibitor of NF-κB (IκB). Proteasome inhibitors have anti-neoplastic properties, at least in part, through blockade of NF-κB activation. Additional anti-neoplastic mechanisms of proteasome inhibitors include interference with degradation of cell cycle regulatory proteins and induction of ER stress-induced apoptosis due to accumulation of misfolded proteins.
Bortezomib
Bortezomib is a reversible proteasome inhibitor that has become a fundamental part of therapy for multiple myeloma, as well as more recent approval for the treatment of relapsed mantle cell lymphoma. The clinical development of bortezomib for PTCL has focused on combination regimens. After a promising phase I study, a phase II trial tested the combination of bortezomib plus CHOP as first-line treatment for 46 patients with advanced-stage PTCL, demonstrating an ORR of 76 %, CR rate of 65 %, and 3-year PFS of 35 % [82]. Another phase I/II trial tested the combination of bortezomib plus gemcitabine for patients with relapsed refractory lymphomas [83]. The regimen was well tolerated when delivered on a bi-monthly schedule; for the six patients with PTCL, the ORR was 50 % with two CRs. As mentioned above, there are numerous ongoing clinical trials testing combinations of bortezomib with novel agents including romidepsin (NCT00963274) and vorinostat (NCT00810576).
Immunomodulators
Lenalidomide
Lenalidomide possesses multifaceted anti-neoplastic properties, functioning as both an immunomodulator and anti-angiogenic factor. Lenalidomide has demonstrated remarkable efficacy in multiple myeloma, follicular lymphoma, DLBCL, chronic lymphocytic leukemia, and MDS with del5q. A phase II trial of lenalidomide for 40 patients with relapsed/refractory PTCL resulted in an ORR of 26 % including 3 CRs, median PFS of 4 months, and median OS of 12 months [84, 85]. Another small trial treated 10 patients with relapsed/refractory PTCL-NOS, demonstrating an ORR of 30 %, all of which were CRs with durations of response ≥1 year [86]. Thus, lenalidomide provides significant benefit to a subset of patients with PTCL, making identification of a biomarker for response a priority. Based on the experience with lenalidomide in other lymphoid malignancies, one might hypothesize that it would exhibit greater efficacy in combination with other agents.
Anti-angiogenic agents
Bevacizumab
Angioimmunoblastic T cell lymphoma is characterized by a vigorous stromal reaction with a prominent vascular component. Accordingly, the pro-angiogenic vascular endothelial growth factor (VEGF) is highly expressed by AITL cells and their surrounding stroma [57, 87]. The fully humanized monoclonal antibody bevacizumab (Avastin) targets VEGF and has been used therapeutically in a number of solid tumors. A potential role for bevacizumab as treatment for PTCL was supported by an anecdotal report describing a complete response to single-agent bevacizumab in a patient with relapsed/refractory AITL [88]. Eventually, a multicenter phase II trial was conducted in which 46 patients with newly diagnosed PTCL were treated with bevacizumab plus CHOP [89]. Despite an ORR of 90 % with 49 % CRs, the remissions were not durable with a median PFS of 7.7 months. Moreover, there was a high rate of grade ≥3 adverse cardiovascular events, including CHF (9 %) and venous thrombosis (7 %) [90].
Kinase inhibitors
Alisertib
Aurora A kinase is a serine/threonine kinase with an essential role in cell division via regulation of sister chromatid separation. Preclinical evidence indicated that Aurora A kinase is overexpressed in non-Hodgkin lymphoma and correlates with inferior survival [91, 92]. Moreover, inhibition of Aurora A kinase can result in cell cycle arrest and apoptosis [92]. Alisertib, also known as MLN8237, is a selective, competitive, reversible inhibitor of Aurora A kinase. A recent phase II trial of alisertib was conducted in patients with relapsed/refractory non-Hodgkin lymphoma, including eight patients with PTCL [93]. The overall response rate was 27 %, but 50 % in the patients with PTCL, including three with CRs lasting over 1 year. A phase III trial is underway comparing alisertib to investigator’s choice in patients with relapsed/refractory peripheral T cell lymphoma (NCT01482962).
Crizotinib
Anaplastic large cell lymphoma can be subdivided based on the expression of the anaplastic lymphoma kinase (ALK), often due to translocations involving the ALK gene [94]. Patients with ALK+ ALCL have the best prognosis amongst all types of PTCL, with a 5-year PFS of >60 % after anthracycline-based combination chemotherapy [10, 95]. Nonetheless, patients with high-risk disease by IPI tend to do poorly, and relapsed/refractory patients need more effective therapies. Crizotinib is a small molecule inhibitor of ALK that demonstrated remarkable efficacy in lung adenocarcinomas possessing ALK translocations. Initial reports described several complete responses to treatment with crizotinib in patients with relapsed ALK+ ALCL [96]. A later series of nine patients with relapsed/refractory ALK+ ALCL lymphoma were treated crizotinib, resulting in an ORR of 100 %, a CR rate of 100 %, median DoR of 10 months, and 3-year PFS of 63 % with a plateau in the curve after 6 months [97]. Interestingly, in the patients who relapsed, secondary mutations in ALK were identified, likely accounting for the acquired resistance to crizotinib.
Moving toward mechanism-based therapy
Important recent advances in understanding the biology of PTCL have resulted in the identification of novel targets that might fundamentally change future treatment paradigms for PTCL. Next-generation sequencing techniques have been used to discover recurrent mutations characteristic of specific PTCL subtypes, many of which were discussed in this review. In another recent example, whole-exome sequencing identified recurrent activating mutations in the SRC family kinase FYN in AITL and PTCL-NOS. Preclinical studies demonstrated that the constitutive activity of mutant FYN could be blocked by the multi-kinase inhibitor dasatanib, creating a rationale for testing this agent in patients with PTCL [98]. Similarly, recurrent activating mutations in JAK3 mutations were identified in natural killer/T cell lymphoma, which might be targetable with the JAK3 inhibitor tofacitinib [99]. Table 3 summarizes additional emerging targets for potential therapeutic intervention.
Complementing the discoveries made by sequencing approaches, gene expression profiling (GEP) and immunohistochemical (IHC) studies identified aberrant activation of oncogenic signaling pathways in the absence of obvious genetic lesions. For example, gene expression profiling and biochemical analysis demonstrated activation of the receptor tyrosine kinase platelet-derived growth factor alpha (PDGFRα) in a significant portion of PTCL-NOS, AITL, and NKTCL [57, 60, 100]. Preclinical studies demonstrated that imatinib, a multi-kinase inhibitor with activity against PDGFRα, could block the growth and survival of T cell lymphoma cell lines as well as a mouse model of T cell lymphoma [60, 101]. These observations provided the rationale for an ongoing pilot study testing imatinib in patients with relapsed/refractory PTCL (NCT00684411).
Another developing story in the biology of PTCL is the importance of the microenvironment, as evidenced by the difficulty generating and growing T cell lymphoma cell lines in vitro. The histologic appearance of many PTCL entities demonstrates extensive infiltration by non-malignant stromal and immune cells. Gene expression profiling of PTCL biopsy specimens demonstrated constitutive activation of cytokine signaling pathways, including NF-κB, IFN/JAK/STAT, and PI3K/AKT/MTOR [57, 59, 60, 100]. These cytokine pathways regulate growth, survival, and proliferation of both normal and malignant lymphocytes, therefore providing promising targets for therapeutic intervention [102–104].
The future success of therapeutic development for peripheral T cell lymphoma will depend on a transition away from histology-driven treatment approaches toward biomarker-driven strategies that apply these new discoveries in PTCL biology. As described in detail throughout this review, most of the therapies that have been tested in relapsed/refractory PTCL represent targeted agents that were given blindly without knowledge of whether the molecular target was present in the patient being treated. These single-agent therapies produced responses, often durable, but only in a subset of patients, suggesting underlying molecular determinants of response. The exceptions prove the rule: brentuximab vedotin and crizotinib each have clearly defined targets and produce dramatic responses as single agents when given to the appropriate patient population (Fig. 2; Table 2).
Identifying biomarkers of response can be achieved through a variety of approaches. At minimum, we believe that all patients with PTCL on clinical trials with single agents should have targeted next-generation sequencing performed on their tumor specimens [105]. Modern techniques make this approach feasible on small quantities of paraffin-embedded tissue from archived clinical lymphoma specimens [106]. Elucidating the mutation profiles of outlier responders can provide important insights into mechanisms of drug sensitivity and resistance that might not have been anticipated a priori [107]. Targeted next-generation sequencing can also be performed on well-annotated, banked specimens from previously completed trials. Retrospective correlation of drug response to genotype will help generate hypotheses to guide the design of future clinical trials.
For example, it will be important to apply targeted next-generation sequencing for PTCL to correlate gene mutation profiles with response to epigenetic-modifying agents. As discussed previously, PTCL-NOS and AITL exhibit recurrent mutations in multiple genes that affect DNA methylation and histone modifications, including TET2, DNMT3A, IDH2, CREBBP, and EP300 [61–63, 65]. It seems likely that patients with different combinations of these mutations will exhibit different responses to HDAC inhibitors, hypomethylating agents, or combinations of these drugs. Identification of genetic determinants of response will ensure that these therapies are only used for patients who will derive benefit from them.
The development of mechanism-based combination strategies will also be critical for future success in treating PTCL, with the ultimate goal of developing chemotherapy-free regimens as has been accomplished in multiple myeloma. In preclinical investigations, histone deacetylase inhibitors have demonstrated striking synergy with a variety of agents, including hypomethylating agents, proteasome inhibitors, PI3K/AKT/MTOR pathway inhibitors, and Aurora A kinase inhibitors [79, 108–111]. These preclinical observations provided the scientific rationale for multiple clinical trials testing HDAC inhibitors in combination with numerous other targeted agents. Another chemotherapy-free combination regimen that will likely be utilized in the near future is brentuximab vedotin plus crizotinib for ALK+ ALCL [47, 97].
Conclusion
The evolution of therapies for peripheral T cell lymphoma can be viewed as an extreme example of the trends seen throughout oncology. Although PTCL was initially considered to be a single disease entity and treated as such, later advances in histopathological classification identified numerous subtypes of PTCL. Nonetheless, the relatively uniform and empiric treatment approach remained unchanged and unsuccessful. Numerous novel agents were tested, but in the absence of identifiable molecular targets, they appeared to benefit only a fraction of patients. Modern advances in biology and genetics resulted in the identification of distinct molecular lesions within and across histological subtypes of PTCL. The current challenge lies in making the leap from empiric therapy to an individualized mechanistic and biomarker-driven treatment approach, based on newly identified molecular targets. Future outcomes for patients with PTCL appear hopeful provided that the proper therapies can be matched to the appropriate patients.
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008.
Vose J, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30.
Morton LM, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–76.
Abouyabis AN, et al. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008;49(11):2099–107.
Federico M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-Cell Lymphoma Project. J Clin Oncol. 2013;31(2):240–6.
Weisenburger DD, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2011;117(12):3402–8.
Gisselbrecht C, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998;92(1):76–82.
Abouyabis AN, et al. A Systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol. 2011;2011:623924.
Reimer P, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106–13.
Savage KJ, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–504.
Mehta N, et al. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin Lymphoma Myeloma Leuk. 2013;13(6):664–70.
Simon A, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151(2):159–66.
Schmitz N, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–25.
Mahadevan D, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119(2):371–9.
d’Amore F, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–9.
Horwitz S, et al. Second-line therapy with ICE followed by high dose therapy and autologous stem cell transplantation for relapsed/refractory peripheral T-cell lymphomas: minimal benefit when analyzed by intent to treat. ASH Annu Meet Abstr. 2005;106(11):2679.
Lunning MA, Moskowitz AJ, Horwitz S. Strategies for relapsed peripheral T-cell lymphoma: the tail that wags the curve. J Clin Oncol. 2013;31(16):1922–7.
Dodero A, et al. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia. 2012;26(3):520–6.
Goldberg JD, et al. Long-term survival in patients with peripheral T-cell non-Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53(6):1124–9.
Le Gouill S, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26(14):2264–71.
Wang ES, et al. Activity of a novel anti-folate (PDX, 10-propargyl 10-deazaaminopterin) against human lymphoma is superior to methotrexate and correlates with tumor RFC-1 gene expression. Leuk Lymphoma. 2003;44(6):1027–35.
O’Connor OA, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol. 2009;27(26):4357–64.
O’Connor OA, et al. Pralatrexate, a novel class of antifol with high affinity for the reduced folate carrier-type 1, produces marked complete and durable remissions in a diversity of chemotherapy refractory cases of T-cell lymphoma. Br J Haematol. 2007;139(3):425–8.
O’Connor OA, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182–9.
Leoni LM, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14(1):309–17.
Rummel MJ, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–10.
Damaj G, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013;31(1):104–10.
Coiffier B, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42.
Pfreundschuh M, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–91.
Yoshie O, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99(5):1505–11.
Ishida T, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10 Pt 1):3625–34.
Ishii T, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–31.
Ishida T, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–42.
Yano H, et al. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: a novel immunotherapy for aggressive/refractory mycosis fungoides and sezary syndrome. Clin Cancer Res. 2007;13(21):6494–500.
Ishida T, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004;10(16):5494–500.
d’Amore F, et al. Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma. Br J Haematol. 2010;150(5):565–73.
O’Mahony D, et al. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clin Cancer Res. 2009;15(7):2514–22.
Piccaluga PP, et al. Expression of CD52 in peripheral T-cell lymphoma. Haematologica. 2007;92(4):566–7.
Jiang L, et al. Variable CD52 expression in mature T cell and NK cell malignancies: implications for alemtuzumab therapy. Br J Haematol. 2009;145(2):173–9.
Enblad G, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920–4.
Gallamini A, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110(7):2316–23.
Kim JG, et al. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother Pharmacol. 2007;60(1):129–34.
Kluin-Nelemans HC, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22(7):1595–600.
Ansell SM, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25(19):2764–9.
Forero-Torres A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–9.
Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21.
Pro B, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–6.
Oki Y, Horwitz S, Bartlett NL, Jacobsen E, Sharman JP, O’Connor OA, Shustov AR, Siddiqi T, Grove LE, Advani R. Safety and efficacy of brentuximab vedotin for treatment of relapsed or refractory mature T-/NK-Cell lymphomas. Proc ICML Abstr. 2013; p. 152.
Fanale MA, et al. Brentuximab vedotin administered concurrently with multi-agent chemotherapy as frontline treatment of ALCL and other CD30-positive mature T-cell and NK-cell lymphomas. ASH Annu Meet Abstr. 2012;120(21):60.
Olsen E, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19(2):376–88.
Prince HM, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(11):1870–7.
Dang NH, et al. Phase II trial of denileukin diftitox for relapsed/refractory T-cell non-Hodgkin lymphoma. Br J Haematol. 2007;136(3):439–47.
Foss FM, et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma. 2013;54(7):1373–9.
Kreitman RJ, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18(8):1622–36.
Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–84.
Fazilleau N, et al. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–35.
Piccaluga PP, et al. Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer Res. 2007;67(22):10703–10.
de Leval L, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109(11):4952–63.
Ballester B, et al. Gene expression profiling identifies molecular subgroups among nodal peripheral T-cell lymphomas. Oncogene. 2006;25(10):1560–70.
Piccaluga PP, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117(3):823–34.
Cairns RA, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119(8):1901–3.
Lemonnier F, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120(7):1466–9.
Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012;366(1):95–6.
Lai AY, Wade PA. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat Rev Cancer. 2011;11(8):588–96.
Odejide O, et al. A targeted mutational landscape of angioimmunoblastic T cell lymphoma. Blood. 2013.
Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67.
Quivoron C, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20(1):25–38.
Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–8.
Moran-Crusio K, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24.
Coiffier B, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631–6.
Piekarz RL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827–34.
Piekarz RL, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27(32):5410–7.
Whittaker SJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(29):4485–91.
O’Connor OA, et al. Belinostat, a novel pan-histone deacetylase inhibitor (HDACi), in relapsed or refractory peripheral T-cell lymphoma (R/R PTCL): Results from the BELIEF trial. ASCO Meeting Abstracts, 2013; 31(15 suppl): p. 8507.
Olsen EA, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(21):3109–15.
Duvic M, et al. Panobinostat activity in both bexarotene-exposed and -naive patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer. 2013;49(2):386–94.
Ellis L, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14(14):4500–10.
Phipps C, et al. Bortezomib and panobinostat combination is effective against PTCL. Leuk Res. 2012;36(6):e128–30.
Marchi E, et al. The combination of hypomethylating agents and histone deacetylase inhibitors (HDACi) are synergistically cytotoxic and reverse the malignant phenotype in preclinical models of T-cell lymphoma. Blood. 2013;122(21):646.
Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–60.
Martinez-Delgado B, et al. Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia. 2005;19(12):2254–63.
Kim SJ, et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: a multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48(17):3223–31.
Evens AM, et al. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol. 2013;163(1):55–61.
Dueck G, et al. Interim report of a phase 2 clinical trial of lenalidomide for T-cell non-Hodgkin lymphoma. Cancer. 2010;116(19):4541–8.
Prasad A, et al. Final report of a phase II clinical trial of lenalidomide monotherapy for T-cell lymphoma. Blood. 2013;122(21):4376.
Zinzani PL, et al. Lenalidomide monotherapy for relapsed/refractory peripheral T-cell lymphoma not otherwise specified. Leuk Lymphoma. 2011;52(8):1585–8.
Zhao WL, et al. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab Invest. 2004;84(11):1512–9.
Bruns I, et al. Complete remission in a patient with relapsed angioimmunoblastic T-cell lymphoma following treatment with bevacizumab. Leukemia. 2005;19(11):1993–5.
Ganjoo K, et al. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2013.
Advani RH, et al. Cardiac toxicity associated with bevacizumab (Avastin) in combination with CHOP chemotherapy for peripheral T cell lymphoma in ECOG 2404 trial. Leuk Lymphoma. 2012;53(4):718–20.
Yakushijin Y, Hamada M, Yasukawa M. The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin’s lymphoma. Leuk Lymphoma. 2004;45(9):1741–6.
Qi W, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81(7):881–90.
Friedberg JW, et al. Phase II study of alisertib, a selective aurora a kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32(1):44–50.
Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1995;267(5196):316–7.
Gascoyne RD, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93(11):3913–21.
Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364(8):775–6.
Farina F, et al. High response rates to crizotinib in advanced, chemoresistant ALK+ lymphoma patients. Blood. 2013;122(21):368.
Couronne L, et al. Activating mutations in fyn kinase in peripheral T-Cell lymphomas. Blood. 2013;122(21):811.
Koo GC, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2(7):591–7.
Huang Y, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115(6):1226–37.
Laimer D, et al. PDGFR blockade is a rational and effective therapy for NPM-ALK-driven lymphomas. Nat Med. 2012;18(11):1699–704.
Maurer MJ, et al. In-vivo activation of STAT3 in angioimmunoblastic T Cell Lymphoma, PTCL not otherwise specified, and ALK negative anaplastic large cell lymphoma: implications for therapy. Blood. 2013;122(21):844.
Juvekar A, et al. Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB–dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res. 2011;9(2):183–94.
Horwitz, SM, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ, γ, in patients with relapsed/refractory lymphoma. ASCO Meeting Abstracts, 2013; 31(15 suppl) p. 8518.
Intlekofer AM, et al. Profiling genomic alterations of diffuse large B-cell lymphoma (DLBCL) at diagnosis, relapse, and transformation, using a novel clinical diagnostic targeted sequencing platform. Blood. 2013;122(21):1761.
Lipson D, et al. Identification of actionable genomic alterations in hematologic malignancies by a clinical next generation sequencing-based assay. Blood. 2013;122(21):230.
Iyer G, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221.
Gupta M, et al. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114(14):2926–35.
Buglio D, et al. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol. 2010;151(4):387–96.
Wozniak MB, et al. Vorinostat interferes with the signaling transduction pathway of T-cell receptor and synergizes with phosphoinositide-3 kinase inhibitors in cutaneous T-cell lymphoma. Haematologica. 2010;95(4):613–21.
Kretzner L, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011;71(11):3912–20.
Rassidakis GZ, et al. BCL-2 family proteins in peripheral T-cell lymphomas: correlation with tumour apoptosis and proliferation. J Pathol. 2003;200(2):240–8.
Chen C, et al. Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev. 2013;27(18):1974–85.
Kim SJ, et al. A phase I study of everolimus and CHOP in newly diagnosed peripheral T-cell lymphomas. Invest New Drugs. 2013;31(6):1514–21.
Souers AJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8.
Wang F, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340(6132):622–6.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Intlekofer, A.M., Younes, A. From empiric to mechanism-based therapy for peripheral T cell lymphoma. Int J Hematol 99, 249–262 (2014). https://doi.org/10.1007/s12185-014-1521-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1521-2