Abstract
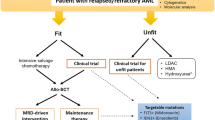
Acute myeloid leukemia (AML) is the most common adult leukemia in Japan. The treatment for AML consists of induction, consolidation, and maintenance therapies. To improve outcomes in the treatment of AML, the Japan Adult Leukemia Study Group has conducted six studies in AML patients aged 15–64 years since 1987. In AML201 study, IDR (12 mg/m2/day for 3 days) or DNR (50 mg/m2/day for 5 days) in combination with Ara-C (100 mg/m2/day continuous infusion for 7 days) was established as the standard induction therapy, and four courses of combination chemotherapy using non-cross-resistant agents for non-core binding factor (CBF) AML or three courses of high-dose Ara-C for CBF AML was established as the standard consolidation therapy. The AML97 study showed that allo-HSCT from an HLA-identical sibling donor reduced relapse incidence and improved disease-free survival (DFS), but did not significantly impact overall survival (OS) in poor or intermediate risk patients. Despite these studies by JALSG, only about one-third of AML patients remain free of disease for more than 7 years. The JALSG is now conducting the AML209 study to adapt individual therapies according to genetic alterations.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a relatively rare cancer, but is the most common leukemia in adults in Japan. AML is a highly heterogeneous disease at multiple levels, including its molecular and genetic background, clinical behavior, and sensitivity to chemotherapy. To conduct a scientific clinical study for the treatment of AML, a large number of patients are required to ensure a reliable statistical analysis. The Japan adult leukemia study group (JALSG) was organized in 1987 to conduct a multicenter cooperative study of AML patients in Japan. Although the JALSG consisted of only 14 institutions at its inception, as of April 2012, it included 119 institutions with 94 affiliated hospitals. Since it was formed, the JALSG has conducted six studies in adult AML patients younger than 65 years old, excluding those with acute promyelocytic leukemia, in an effort to establish a standard therapeutic regimen. I herein summarize the JALSG studies of AML, focusing on two trials, AML97 and AML201.
Induction therapy
For more than three decades, the combination of anthracycline and cytarabine (Ara-C) has been a standard induction therapy for AML, and a combination of daunorubicin (DNR) at a dose of 45–50 mg/m2/day for 3 days and Ara-C at a dose of 100–200 mg/m2/day given by continuous infusion for 7 days has generally been used. Many studies have been conducted to attempt to improve the remission rate and to achieve deeper remission. Studies using high-dose Ara-C (HiDAC) found no improvement of the CR rate and a high incidence of treatment-related toxicities [1, 2]. In the late 1980s, however, idarubicin (IDR) was introduced into clinics, and three randomized studies comparing IDR with DNR reported significantly higher CR rates for IDR [3–5]. Furthermore, a meta-analysis also confirmed that IDR at a dose of 10–12 mg/m2/day for 3 days had a superior effect than DNR at a dose of 45–60 mg/m2/day for 3 days in the achievement of a CR [6]. For this reason, IDR and Ara-C were used as induction therapy in the AML95 [7] and AML97 studies [8]. Both studies yielded CR rates similar to those in the earlier AML87 [9], AML89 [10], and AML92 [11] studies. In these three studies, DNR and other drugs were administered in a response-oriented individualized manner; i.e., additional drugs were given for a few days when the bone marrow at day 8 was not hypoplastic and contained a substantial number of blasts. Therefore, the total doses of DNR administered during the first course of induction therapy were 240–280 mg/m2 given for more than 5–7 days, which was more than the conventional dose of 40–60 mg/m2 given for 3 days. As there had been no direct comparison between a high dose of DNR and the standard dose of IDR (12 mg/m2), we prospectively compared IDR (12 mg/m2 for 3 days) with DNR (50 mg/m2 for 5 days), in combination with Ara-C (100 mg/m2 for 7 days), in the AML201 study [12]. Of the 1064 patients registered, 1057 patients were evaluable. They were randomly assigned to receive either DNR or IDR. The CR rate was 77.5 % (95 % CI 73.8–80.9 %) for the DNR group and 78.2 % (95 % CI, 74.5 %–81.5 %) for the IDR group (P = 0.79). These results showed that the high-dose DNR regimen was not inferior to the IDR regimen. In addition, the 5-year OS was 48 % for the DNR group and 48 % for the IDR group (P = 0.54), and the 5-year relapse-free survival (RFS) rates were 41 and 41 % (P = 0.97), respectively. Consequently, both regimens produced a high CR rate and had good long-term efficacy. However, sepsis occurred more frequently in the IDR group than in the DNR group (8.7 and 4.9 %, P = 0.02) and early death within 60 days occurred more frequently in the IDR group than in the DNR group (4.7 and 2.1 %, P = 0.03). Acute and late-onset cardiotoxicities were reported only in a small number of patients in both groups. We concluded that both regimens can be used, and they are now the standard induction therapy in Japan at this time. Recently, The Eastern Cooperative Oncology Group also reported that a higher dose of DNR (90 mg/m2 for 3 days) improved the CR rate and OS, as were seen in JALSG studies [13]. Given this and JALSG reports, the optimal total dose of DNR is still to be explored but may rest somewhere between 250 and 270 mg/m2.
To identify a better induction therapy for AML, we conducted a phase I trial (AML206) of a combination of gemtuzumab ozogamicin (GO) with the standard induction therapies for patients with relapsed/refractory AML. This study showed that 3 mg/m2 GO combined with Ara-C and the standard dose of IDR or the high dose of DNR can be safely administered [14]. We were planning a phase II trials to investigate the clinical efficacy of this combination therapy, but GO was withdrawn from the US market in 2010 based on data from the Southwest Oncology Group (SWOG) Study S0106, which failed to confirm a clinical benefit and showed a high rate of fatal induction toxicity. More recently, however, the outcomes of the Acute Leukemia French Association (ALFA)-0701 study have been reported [15]. These results showed that the addition of fractionated lower doses (3 mg/m2) of GO to the standard chemotherapy allows the safe delivery of higher cumulative doses and substantially improves CR rate and OS. The optimal dose and schedule should be decided in future trials by leukemia study groups, including the JALSG.
Consolidation chemotherapy
Consolidation therapy is generally given immediately after a patient achieves CR, and its intensity is the same as that for induction therapy. Although intensive consolidation therapy is necessary for curative results, the optimal type and number of cycles have yet to be defined. HiDAC therapy is generally used in the United States and other countries after the landmark Cancer and Leukemia Group B-8525 (CALGB-8525) study [16]. In Japan, however, because HiDAC therapy was not approved by our national medical insurance system until 2001, a standard dose of Ara-C-based combination chemotherapy using non-cross-resistant agents (multiagent CT) was commonly used in early the five studies of adult AML performed by the JALSG. Therefore, we more recently compared conventional multiagent CT with HiDAC therapy in he AML201 trial [17]. In this trial, 781 CR patients were randomly assigned to receive either the HiDAC regimen or the multiagent CT regimen. The 5-year DFS was 43 % for the HiDAC group and 39 % for the multiagent CT group, and the 5-year OS rates were 58 and 56 %, respectively. There were therefore no significant differences in the DFS and OS between the groups. However, among the favorable cytogenetic risk group (n = 218), the 5-year DFS was 57 % for the HiDAC and 39 % for multiagent CT (P = 0.050) (Fig. 1), but the 5-year OS rates were 75 and 66 %, respectively (P = 0.174). After each course of consolidation, the nadir of the WBC count was significantly lower and the day when a WBC less than 1.0 × 109/L was noted was significantly longer in the HiDAC group. The frequency of documented infections was also significantly higher in the HiDAC group, even though the frequency and the number of days of granulocyte colony-stimulating factor (G-CSF) administration were significantly higher in the HiDAC group. However, the subset analysis showed that the higher incidence of documented infection following treatment with the HiDAC regimen was noted only in the intermediate cytogenetic risk group. Death in CR was reported in only a small number of patients in both groups. Therefore, HiDAC therapy can be recommended only for patients with favorable cytogenetics. This limitation of HiDAC therapy is associated with lower medical expenses, because HiDAC therapy and granulocyte colony-stimulating factor administration are costly.
Relapse-free survival by treatment arm and karyotype risk. a Relapse-free survival of CR patients by treatment arm. Predicted 5-year RFS was 43 % for the HiDAC group (n = 389) (red line) and 39 % for the standard dose group (n = 392) (blue line) (P = 0.724). b Disease-free survival by treatment arm for the favorable cytogenetic risk group. Predicted 5-year DFS was 57 % for the HiDAC group (n = 108) (red line) and 39 % for the multiagent CT group (n = 110) (blue line) (P = 0.050). c Disease-free survival by treatment arm for the intermediate cytogenetic risk group. Predicted 5-year DFS was 38 % for the HiDAC group (n = 242) (red line) and 39 % for the multiagent CT group (n = 256) (blue line) (P = 0.403). d Disease-free survival and overall survival by treatment arm for the adverse cytogenetic risk group. Predicted 5-year DFS was 33 % for the HiDAC group (n = 27) (red line) and 14 % for the multiagent CT group (n = 14) (blue line) (P = 0.364)
Hematopoietic stem cell transplantation as consolidation therapy
Allo-HSCT is the most intensive treatment option for AML, but the low relapse rate is usually offset by a high incidence of treatment-related mortality (TRM). Many clinical trials have compared allo-HSCT with chemotherapy as a post-remission therapy for the patients with AML in CR. Some studies have not found a benefit of allo-HSCT on either the DFS or OS [18, 19], and some showed an advantage of allo-HSCT only on the DFS when it was compared with chemotherapy/auto-transplantation [20, 21]. Most of these studies targeted all patients in CR as a single population, without prospective stratification by their prognostic factors. We prospectively compared the effectiveness of allo-HSCT with chemotherapy among CR patients aged 15–50 years, who were stratified into intermediate- or poor risk groups following the JALSG scoring system in the AML97 study [22]. Seventy-three CR patients with and 92 without an HLA-identical sibling were assigned to the donor and non-donor groups. Of the 73 patients in the donor group, 38 (52 %) received allo-HSCT during the first CR and 17 (23 %) after relapse. An intention-to-treat analysis revealed that the relapse incidence was reduced in the donor group (52 vs. 77 %; P = 0.008), and that the DFS was improved (39 vs. 19 %; P = 0.016), but the OS was not significantly different (46 vs. 29 %; P = 0.088). However, an OS benefit was seen in the patients aged 36–50 years (49 vs. 24 %; P = 0.031). Therefore, it is reasonable that allo-HSCT should be performed for intermediate or poor risk patients with an HLA-identical donor when they are in CR.
Maintenance therapy
In contrast to consolidation therapy, maintenance therapy is of low intensity and is prolonged, but the benefits of such therapy remain unclear. The AML87 trial [9] showed that a significantly longer DFS was achieved when 12 courses of moderately intensive maintenance therapy were administered instead of the four courses of maintenance therapy. However, to preserve the quality of life of the patients in the following AML trials (AML89 [10], AML92 [11], and AML95 [7]), six courses of maintenance therapy were used, and demonstrated a better OS than that achieved in the AML87 study. Six courses of maintenance therapy became the standard regimen in Japan thereafter. We conducted the AML97 trial [8] to compare four courses of standard-dose consolidation therapy without maintenance (Arm A) with three courses of consolidation therapy followed by six courses of maintenance therapy (Arm B). In the AML97 trial, 998 CR patients were assigned randomly to either Arm A or Arm B. The 5-year OS rate was 52.4 % for Arm A and 58.4 % for Arm B (P = 0.599), and the 5-year DFS rates were 35.8 and 30.4 % (P = 0.543), respectively. In analyzing the data by risk group, no statistical difference was observed either in the 5-year OS or in the 5-year DFS between the two arms. We concluded that prolonged consolidation and maintenance therapy could be successfully achieved with four courses of consolidation therapy in all AML risk groups. Considering the results of the AML87 [9] and AML97 [8] trial, the benefits of maintenance therapy seemed to be dependent on the intensity of the consolidation given prior to the maintenance therapy. Given the recent use of intensive consolidation, maintenance therapy is therefore considered to be unnecessary. Moreover, avoiding the use of maintenance therapy improves the quality life of adult AML patients.
Salvage therapy
Over 50 % of CR patients eventually relapse within 3 years, despite intensive post-remission chemotherapy. Considering that relapsed AML is not curable by treatment with chemotherapy alone, and that the outcome after allo-HSCT in the second CR is comparable to that after transplantation in the first CR, salvage therapy followed by allo-HSCT is imperative to increase the cure rate for patients with AML. HiDAC is one of the promising salvage therapies, but its efficacy for early relapsed patients is limited. Based on the finding that fludarabine augments the accumulation of Ara-C-triphosphate, investigators at the M. D. Anderson Cancer Center developed a combination therapy with fludarabine, HiDAC and G-SCF (FLAG) [23]. We developed a FLAGM regimen by adding mitoxantrone to FLAG. After determining the safe dosage of the combination therapy in a phase I study [24], we performed a phase II study using FLAGM for relapsed/refractory adult AML patients. Forty-one patients aged 18–64 years (median 52) enrolled in this trial. The FLAGM regimen yielded a high CR rate (73 %) with a relatively low toxicity profile, regardless of the duration of the first CR or the preceding HiDAC regimen, and it appears to be a good salvage therapy for relapsed/refractory AML patients, which enables a high proportion of patients (80 %) to undergo allo-HSCT [25]. Therefore, our FLAGM regimen represents a promising salvage regimen for relapsed/refractory AML in young adults. The JALSG is planning to conduct a large-scale prospective study with FLAGM.
Prognostic factors
Prognostic factors are important not only for predicting prognosis, but also for stratifying patients to determine optimal treatment. This is especially true because of the heterogeneity of AML. Cytogenetics is one of the most important prognostic factors. The karyotype of the leukemic cells can be divided into three groups categorized as favorable, intermediate, and poor risk, based on the specific karyotype [26, 27].
It has recently been reported that mutations or overexpression of specific genes have prognostic significance [28]. In core binding factor (CBF) AML [e.g. AML with t(8;21) or inv(16)], mutations in KIT have been shown to have a potentially unfavorable influence. In cytogenetically normal AML (CN-AML), which is identified in about 50 % of patients within intermediate risk, fms-like tyrosine kinase 3 gene-internal tandem duplications (FLT3-ITD) [29], CCAAT/enhancer binding protein alpha (CEBPA), and nucleophosmin1 (NPM1) mutations are sometimes identified alone or in combination. FLT3-ITD is associated with unfavorable outcome, while CEBPA and NPM1 are associated with favorable outcome if present without FLT3-ITD. The European leukemiaNet therefore proposed a new classification that includes data from a cytogenetic analysis and from a mutation analysis of the NPM1, CEBPA, and FLT3 genes [30]. High expression of the brain and acute leukemia cytoplasmic (BAALC) gene and the ETS-related gene (ERG) has a negative impact on the prognosis of patients with CN-AML.
The JALSG developed a different scoring system (JALSG scoring system) using the karyotype, age, WBC count at presentation, FAB classification, positivity for peroxidase in blasts, performance status (PS), number of induction therapies required to achieve complete remission (CR), which were extracted for achieving CR, DFS, and overall survival (OS) using a multivariate analysis of the results from three studies (AML87 [9], AML89 [10], AML92 [11]) [31]. We prospectively applied the JALSG scoring system to stratify patients in the AML97 study [22], in which allogeneic hematopoietic stem cell transplantation (allo-HSCT) was assigned as consolidation therapy to those with an intermediate or poor risk classification. The OS and DFS of the CR patients in the AML97 study were clearly divided into three groups by the JALSG scoring system. Furthermore, it was shown that the JALSG scoring system more efficiently selected poor responders to chemotherapy in the favorable risk group, and good or poor responders to chemotherapy in the intermediate risk group compared to karyotype-based stratification [32]. We are now planning to create a new JALSG scoring system based on the clinical data from the AML201 study, in which more than 1000 AML patients were enrolled, as the results of the gene mutation analyses are not clinically available.
Conclusion
The JALSG has conducted six randomized, phase III multicenter trials in the 25 years since its formation, and has established the standard induction and post-remission therapy in Japan. The results of these trials are summarized in Table 1 and Fig. 2. Unfortunately, the CR rate has not changed significantly, and the 7-year OS rates in all of the studies, except for the AML201 trial, have ranges from 28.6 to 36.2 % (Table 1; Fig. 1). However, the OS in the AML201 trial was markedly improved, reaching 48.0 %. Table 2 shows the frequency of allo-HSCT in each trial. Over time, allo-HSCT has gradually increased. Notably, about 50 % of the patients enrolled in the AML201 trial underwent allo-HSCT. Considering these data, it is highly possible that the improvement of the OS in the AML201 trial resulted from the large number of patients who underwent allo-HSCT at the primary induction failure and after relapse. These data not only suggest that allo-HSCT provides an OS benefit, but also that our chemotherapy approaches have reached the limit of their efficacy for AML in young adults.
To further improve the prognosis of young adult AML patients, given that many genomic alterations in AML have been demonstrated to influence the response to clinical therapies, further efforts to integrate genome-wide molecular alterations with the treatment response and outcome are needed. The JALSG is now conducting the AML209 Genomic Study (AML209-GS) (UMIN 000003432) to clarify the genotypes of patients so that they can be stratified for individual therapies by prospectively and comprehensively analyzing the genetic alterations in 1500 newly diagnosed AML patients. In the AML209-GS study, the AML cells obtained from registered patients are currently being subjected to the analyses of cytogenetics and comprehensive gene mutations such as those of FLT3, NPM1, CEBPA, MLL-PTD, KIT, NRAS, TP53, WT1 and IDH. Furthermore, to evaluate the efficacy and safety of the individual therapies based on the FLT3-ITD or KIT mutation status, the JALSG is conducting the AML209-FLT3-SCT and CBF-AML209-KIT studies. Patients carrying a mutation in FLT3-ITD who achieve CR and are younger than 50 years old are registered in the AML209-FLT3-SCT study, and will receive allo-SCT during the 1st CR. The CBF-AML patients who achieve CR are registered in the CBF-AML20-KIT study, and will be treated with three courses of HiDAC. In this study, three types of KIT mutations are being analyzed and evaluated for their prognostic impacts.
More recently, the JALSG AML209 genome-wide study (AML209-GWS) has been initiated as an accompanying study for the AML209-GS study, in which whole exon or genome sequences, as well as the methylome of AML cells, will be examined to identify the genetic and/or epigenetic status associated with the long-term prognosis and the sensitivity to and adverse events from chemotherapies. These JALSG studies are expected to establish a novel stratification system for treatment decision making, providing a more personalized therapy for Japanese AML patients. We are hopeful that a large number of patients can be recruited, and that these studies can be successfully accomplished in the short-term.
References
Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–7.
Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996;88:2841–51.
Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, et al. Results of randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666–74.
Wiernik PH, Banks PL, Case DC Jr, Arlin ZA, Periman PO, Todd MB, et al. Cytarabine idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–9.
Vogler WR, Velez-Garcia E, Weiner RS, Flaum MA, Bartolucci AA, Omura GA, et al. A phase III trial comparing idarubicin and daunorubicin with in acute myeloid leukemia: a Southeastern Cancer Study Group study. J Clin Oncol. 1992;10:1103–8.
The AML collaborative Group. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction for acute myeloid leukemia. Br J Haematol. 1998;103:100–9.
Ohtake S, Miyawaki S, Kiyoi H, Miyazaki Y, Okumura H, Matsuda S, et al. Randomized trial of response-oriented individualized versus fixed-schedule induction chemotherapy with idarubicin and cytarabine in adult acute myeloid leukemia: the JALSG AML95 study. Int J Hematol. 2010;91:276–83.
Miyawaki S, Sakamaki H, Ohtake S, Emi N, Yagasaki F, Mitani K, Matsuda S, et al. A randomized, postremission comparison of four courses of standard-dose consolidation therapy without maintenance therapy versus three courses of standard-dose consolidation with maintenance therapy in adults with acute myeloid leukemia. Cancer. 2005;104:2726–34.
Ohno R, Kobayashi T, Tanimoto M, Hiraoka A, Imai K, Asou N, et al. Randomized study of individualized induction therapy with or without vincristine, and of maintenance intensification therapy between 4 or 12 courses in adult acute myeloid leukemia: AML-87 Study of the Japan Adult Leukemia Study Group. Cancer. 1993;71:3888–95.
Kobayashi T, Miyawaki S, Tanimoto M, Kuriyama K, Murakami H, Yoshida M, et al. Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance/intensification therapy in adult acute myeloid leukemia. J Clin Oncol. 1996;14:204–13.
Miyawaki S, Tanimoto M, Kobayashi T, Kuriyama K, Murakami H, Yoshida M, et al. No beneficial effect from addition of etoposide to daunorubicin, cytarabine, and 6-mercaptopurine in individualized induction therapy of adult acute myeloid leukemia: the JALSG-AML92 study. Int J Hematol. 1999;70:97–104.
Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSGAML201 Study. Blood. 2011;117:1613–8.
Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–59.
Usui N, Takeshita A, Nakaseko C, Dobashi N, Fujita Hi, Kiyoi H, et al. Phase I trial of gemtuzumab ozogamicin in intensive combination chemotherapy for relapsed or refractory adult acute myeloid leukemia (AML): Japan Adult Leukemia Study Group (JALSG)-AML206 study. Cancer Sci. 2011;102:1358–65.
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16.
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331(14):896–903.
Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, Sakura T, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117:2366–72.
Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649–56.
Harousseau JL, Cahn JY, Pignon B, et al. Comparison of autologous bone marrow transplantation and intensive chemotherapy as postremission therapy in adult acute myeloid leukemia. The Groupe Ouest Est Leucemies Aigues Myeloblastiques (GOELAM). Blood. 1997;90:2978–86.
Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118:385–400.
Keating S, de Witte T, Suciu S, et al. The influence of HLA matched sibling donor availability on treatment outcome for patients with AML: an analysis of the AML 8A study of the EORTC Leukaemia Cooperative Group and GIMEMA. European Organization for Research and Treatment of Cancer. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol. 1998;102(5):1344–53.
Sakamaki H, Miyawaki S, Ohtake S, Emi N, Yagasaki F, Mitani K, et al. Allogeneic stem cell transplantation versus chemotherapy as post-remission therapy for intermediate or poor risk adult acute myeloid leukemia: results of the JALSG AML97 study. Int J Hematol. 2010;91:284–92.
Estey E, Plunkett W, Gandhi V, Rios MB, Kantarjian H, Keating MJ. Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk Lymphoma. 1993;9:343–50.
Miyawaki S, Kawai Y, Takeshita A, Komatsu N, Usui N, Arai Y, et al. Phase I trial of FLAGM with high doses of cytosine arabinoside for relapsed, refractory acute myeloid leukemia: study of the Japan Adult Leukemia Study Group (JALSG). Int J Hematol. 2007;86:343–7.
Hatsumi N, Miyawaki S, Yamauchi T, Takeshita A, Komatsu N, Usui N, et al. Phase II Study of FLAGM (fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor + mitoxantrone) for relapsed or refractory acute myeloid leukemia: Recommendable salvage therapy for subsequent stem cell transplantation. Leuk Lymphoma. 2012 Submitted.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–83.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33.
Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18.
Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Kuriyama K, Tomonaga M, Kobayashi T, et al. Trial to extract prognostic factors prior to the start of induction chemotherapy for adult AML. In: Hiddeman W, Buchner T, Worman B, et al., editors. Acute leukemias VII: experimental approaches and novel therapies. New York: Springer; 1998. p. 901–905.
Miyazaki Y, Nishida K, Kuriyama K, Taniwaki M, Sakamaki H, Miyawaki S, et al. A new scoring system to predict the prognosis of patients with acute myeloid leukemia. study from the Japan Adult Leukemia Study Group. Blood. 2005;106:667a. Abstract 2373.
Acknowledgments
The author thanks Shigeki Ohtake, who is the head of the data center of the JALSG, for providing the tables and figures. The author also thanks Tomoki Naoe, who is the president of JALSG, and Hitoshi Kiyoi, who is the principal investigator of the AML209 study, for advice on this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Miyawaki, S. Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 96, 171–177 (2012). https://doi.org/10.1007/s12185-012-1150-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1150-6