Abstract
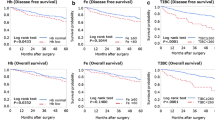
We conducted a retrospective study to find out the optimum values of serum ferritin and other hematologic indices in adult anemic patients who should be referred for thorough gastrointestinal (GI) endoscopic evaluation for GI neoplasms. 544 adult anemic patients were stratified into three groups according to the results of GI endoscopy: benign versus premalignant versus malignant. As compared to non-malignant groups, malignant group demonstrated statistically significant differences in terms of median values of ferritin and total iron-binding capacity (TIBC) saturation. By receiver operating characteristics curve analyses to find out optimum cut-off points of the serum ferritin and TIBC saturation which distinguish between non-malignant diseases and malignant diseases, the cut-off ferritin value of 44.33 ng/mL in male had 72.73 % sensitivity and 70.95 % specificity. The cut-off TIBC saturation value of 9.13 % in male had 73.33 % sensitivity and 70.92 % specificity. The cut-off TIBC saturation value of 6.16 % in female had 69.57 % sensitivity and 65.13 % specificity. It is recommended that adult male patients with anemia undergo thorough endoscopic evaluation to detect GI neoplasms when their serum ferritin levels are ≤44 ng/mL or TIBC saturation values are ≤9 %. For adult female, only TIBC saturation values less than 6 % may contribute to determining whether they undergo GI endoscopic evaluation.


Similar content being viewed by others
References
Blanc B, Finch CA, Hallberg L. Nutritional anemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405:1–40.
Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145–53.
Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–7.
Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: a population-based cohort study. Am J Med. 2002;113:276–80.
Wang SA, Fadare O, Nagar A, Shafi NQ, Rose MG. Gastrointestinal endoscopic findings in men with unexplained anemia and low normal ferritin values. Am J Hematol. 2006;81:324–7.
Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93.
Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41.
Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329:1691–5.
Kepczyk T, Kadakia SC. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Dig Dis Sci. 1995;40:1283–9.
Patterson RN, Johnston SD. Iron deficiency anaemia: are the British Society of Gastroenterology guidelines being adhered to? Postgrad Med J. 2003;79:226–8.
Sawhney MS, Lipato T, Nelson DB, Lederle FA, Rector TS, Bond JH. Should patients with anemia and low normal or normal serum ferritin undergo colonoscopy? Am J Gastroenterol. 2007;102:82–8.
Hardwick RH, Armstrong CP. Synchronous upper and lower gastrointestinal endoscopy is an effective method of investigating iron-deficiency anaemia. Br J Surg. 1997;84:1725–8.
Adamson JW. Iron deficiency and other hypoproliferative anemias. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison’s principles of internal medicine. USA: McGraw-Hill Companies, Inc.; 2008. p. 628–34.
Casale G, Bonora C, Migliavacca A, Zurita IE, de Nicola P. Serum ferritin and ageing. Age Ageing. 1981;10:119–22.
Zanella A, Gridelli L, Berzuini A, Colotti MT, Mozzi F, Milani S, et al. Sensitivity and predictive value of serum ferritin and free erythrocyte protoporphyrin for iron deficiency. J Lab Clin Med. 1989;113:73–8.
Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51.
Birgegard G, Hallgren R, Killander A, Stromberg A, Venge P, Wide L. Serum ferritin during infection. A longitudinal study. Scand J Haematol. 1978;21:333–40.
Hansen TM, Hansen NE. Serum ferritin as indicator of iron responsive anaemia in patients with rheumatoid arthritis. Ann Rheum Dis. 1986;45:596–602.
Huang D, Sumegi J. Dal Cin P, Reith JD, Yasuda T, Nelson M, et al. C11orf95-MKL2 is the resulting fusion oncogene of t(11;16)(q13;p13) in chondroid lipoma. Genes Chromosomes Cancer. 2010;49:810–8.
Jacobs A, Worwood M. Ferritin in serum. Clinical and biochemical implications. N Engl J Med. 1975;292:951–6.
Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver disease. J Intern Med. 1994;236:315–22.
Keane C, Mollee P, Marlton P, Gill D. Treatment of acute promyelocytic leukaemia in the Jehovah’s Witness population. Ann Hematol. 2010;90:359–60.
Matzner Y, Konijn AM, Hershko C. Serum ferritin in hematologic malignancies. Am J Hematol. 1980;9:13–22.
Tran TN, Eubanks SK, Schaffer KJ, Zhou CY, Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997;90:4979–86.
Kis AM, Carnes M. Detecting iron deficiency in anemic patients with concomitant medical problems. J Gen Intern Med. 1998;13:455–61.
Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–64.
Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345–52.
Kobune M, Miyanishi K, Takada K, Kawano Y, Nagashima H, Kikuchi S, et al. Establishment of a simple test for iron absorption from the gastrointestinal tract. Int J Hematol. 2011;93:715–9.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lee, M.H., Park, E., Lee, J. et al. Cutoff values of serum ferritin and TIBC saturation for the evaluation of gastrointestinal neoplasms in adult anemic patients. Int J Hematol 96, 214–221 (2012). https://doi.org/10.1007/s12185-012-1129-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1129-3