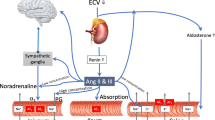
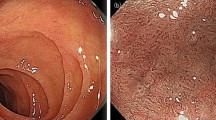
Abstract
Olmesartan-associated enteropathy (OAE) is a newly described condition reported in several case series in which patients taking olmesartan developed diarrhea, nausea, vomiting, dehydration, and weight loss. Although the symptoms and histologic findings of small-bowel enteropathy resembled severe celiac disease, laboratory work and the lack of response to a gluten-free diet challenged that diagnosis. The injury extended beyond the small bowel, with evidence of lymphocytic/collagenous gastritis and/or colitis in a substantial subset of patients. After a thorough diagnostic evaluation including consideration of alternate diagnoses, and resistance to a variety of treatments, a common thread became apparent: that all affected patients were taking olmesartan. Once this connection was recognized and the medication was suspended, symptoms would improve and the enteropathy healed. Some patients required corticosteroids particularly budesonide (a topically potent steroid) to achieve remission. There remains a gap in knowledge regarding the predisposing factors and mechanism of action.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Boscá MM, Añón R, Mayordomo E, et al. Methotrexate induced sprue-like syndrome. World J Gastroenterol. 2008;14:7009–11.
Weclawiak H, Ould-Mohamed A, Bournet B, et al. Duodenal villous atrophy: a cause of chronic diarrhea after solid-organ transplantation. Am J Transplant. 2011;11:575–82.
Brunner HR. The new oral angiotensin II antagonist olmesartan medoxomil: a concise overview. J Hum Hypertens. 2002;16(Supplement 2):S13–6.
Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732–8. This publication first described the clinical and histologic features of olmesartan-associated enteropathy in a case series of 22 patients seen at the Mayo Clinic Rochester, MN between 2008 and 2011.
Stanich P, Yearsley M, Meyer M. Olmesartan-associated sprue-like enteropathy. J Clin Gastroenterol. 2013;47(10):894–5.
Dreifuss SE, Tomizawa Y, Farber NJ, et al. Spruelike enteropathy associated with olmesartan: an unusual case of severe diarrhea. Case Rep Gastrointest Med. 2013;2013:618071.
Nunge D, Eoche M, Fumery M, et al. Entéropathie sévère avec atrophie villositaire associée à la prise d’olmésartan médoxomil. Therapie. 2013;68(6):419–21.
Fiorucci G, Puxeddu E, Colella R, et al. Severe spruelike enteropathy due to olmesartan. Rev Esp Enferm Dig. 2014;106(2):142–4.
Gaur V, Albeldawi M, Weber L, et al. Chronic diarrhea and weight loss. Gastroenterology. 2014;146:591.
Théophile H, David XR, Miremont-Salame G, et al. Five cases of sprue-like enteropathy in patients treated by olmesartan. Dig Liver Dis. 2014;46:465–9. This small case series describes five patients with olmesartan associated enteropathy from a small French gastroenterology unit. One patient had colitis. The authors suggest that the adverse event may not be as rare as initially thought.
Hartranft ME, Regal RE. “Triple Phase” budesonide capsules for the treatment of olmesartan-induced enteropathy. Ann Pharmacother 2014;48(9):1234–1237.
Nielsen JA, Steephen A, Lewin M. Angiotensin II inhibitor (olmesartan)-induced collagenous sprue with resolution following discontinuation of the drug. World J Gastroenterol. 2013;19:6928–30.
Tran TH, Li H. Olmesartan and drug-induced enteropathy. Pharm Ther. 2014;39:47–50.
Khan AS, Peter S, Wilcom CM. Olmesartan-induced enteropathy resembling celiac disease. Endoscopy. 2014;46(S01):E97–8.
Van Beurden YH, Nijeboer P, Janssen J, et al. Diarrhoea and malabsorption due to olmesartan use. Ned Tijdschr Geneeskd. 2014;158:A7370.
Agudo Fernández S. Sprue-like enteropathy due to olmesartan: a case report. Gastroenterol Hepatol. 2014. doi:10.1016/j.gastrohep.2014.05.006.
Téllez A, Pellicé M, Llobell A, Milisenda JC. Enteropathy associated with chronic use of olmesartan. Med Clin (Barc). 2014. doi:10.1016/j.medcli.2014.03.031.
Basson M, Mezzarobba M, Weill A, et al. Severe malabsorption associated with olmesartan: a French nationwide cohort study. Chicago: Oral presentation at: Digestive Disease Week; 2014. p. 3–6. This large cohort study found that the development of OAE significantly correlated with duration of exposure. The risk increased with one year of exposure and further increased thereafter.
Marthey L, Cadiot G, Seksik P, et al. Olmesartan-associated sprue: a French national study. Chicago: Poster session presented at: Digestive Disease Week; 2014. p. 3–6.
Sanford ML, Nagel AK. A review of current evidence of olmesartan medoximil mimicking symptoms of celiac disease. J Pharm Pract. 2014;28. The authors note the recent case reports and series of patients with olmesartan associated enteropathy. They emphasize the need for larger, randomized trials to determine if a causal relationship exists.
Ianiro G, Bibbò S, Montalto M, et al. Systemic review: sprue-like enteropathy associated with olmesartan. Aliment Pharmacol Ther. 2014;40(1):16–23. The authors reviewed 54 cases, including 2 from their series, with olmesartan associated enteropathy. Patients commonly had normocytic anemia and hypoalbuminemia.
Abdelghany M, Gonzalez 3rd L, Slater J, et al. Olmesartan associated sprue-like enteropathy and colon perforation. Case Rep Gastrointest Med. 2014;2014:494098.
Rubio-Tapia A, Talley NJ, Gurudu ST, et al. Gluten-free diet and steroid treatment are effective therapy for most patients with collagenous sprue. Clin Gastroenterol Hepatol. 2010;8(4):344–9. First paper that recognized an increase of collagenous sprue cases and olmesartan use.
Goel A, Lebwohl B, Tennyson C, et al. Histopathologic outcomes in olmesartan related enteropathy. Chicago: Poster session presented at: Digestive Disease Week; 2014. p. 3–6.
Gentile NM, D’Souza A, Fujii LL, et al. Association between ipilimumab and celiac disease. Mayo Clin Proc. 2013;88(4):414–7.
Mini-Sentinel. Modular program report: angiotensin II receptor blockers & celiac disease. 2012. (Accessed April 5, 2012, at http://www.mini-sentinel.org/work_products/Assessments/Mini-Sentinel_Angiotensin-II-Receptor-Blockers-and-Celiac-Disease.pdf).
Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307(5717):1920–5.
Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, et al. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–94.
Nagib MM, Tadros MG, ElSayed MI, Khalifa AE. Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicol Appl Pharmacol. 2013;271(1):106–13.
Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–8.
De Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335(14):1016–21.
DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac disease serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647–53.
Compliance with Ethics Guidelines
Conflict of Interest
Joseph Murray and Amanda Cartee have no disclosures relevant to this work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Hypertension
Rights and permissions
About this article
Cite this article
Cartee, A.K., Murray, J.A. Sprue-like Enteropathy Associated with Olmesartan. Curr Cardiovasc Risk Rep 8, 420 (2014). https://doi.org/10.1007/s12170-014-0420-7
Published:
DOI: https://doi.org/10.1007/s12170-014-0420-7