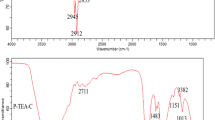
Abstract
Sodium dodecyl sulfate (SDS)/room temperature ionic liquids (RTILs) 1-hexyl-3-methylimidazolium bromide aqueous two-phase systems (ATPSs) are presented as separation/enrichment coupled with ultraviolet spectrometry for separation/analysis safranine T in food samples. The main factors affecting the ATPSs, such as amount of SDS, RTILs, pH, and time, have been investigated in detail. Under the optimal conditions, the linear calibration curves for safranine T was obtained over the concentration ranges of 0.05–4.0 μg mL−1 with the correlation coefficient (R) 0.9984 and the detection limits of safranine T was 3.8 ng mL−1. The mechanism of ATPS phase separation for safranine T has been discussed. The method was successfully applied to the determination of safranine T in food samples.




Similar content being viewed by others
References
Ahmaruzzaman M, Gupta VK (2011) Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind Eng Chem Res 50(24):3589–13613
Asfaram A, Ghaedi M, Goudarzi A, Soylak M (2015) Comparison between dispersive liquid–liquid microextraction and ultrasound-assisted nanoparticles-dispersive solid-phase microextraction combined with microvolume spectrophotometry method for the determination of Auramine-O in water samples. RSC Adv 5(49):39084–39096
Babu BR, Rastogi NK, Raghavarao KSMS (2008) Liquid–liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem Eng Process 47(1):83–89
Bulgariu L, Bulgariu D (2006) Hg (II) extraction in a PEG-based aqueous two-phase system in the presence of halide ions I liquid phase analysis. Cent Eur J Chem 4(2):246–257
Cao Y, He XW (1998) Studies of interaction between safranine T and double helix DNA by spectral methods. Spectrochim Acta A 54(6):883–892
Cheng YH, Li QB (2010) Determination of trace copper (II) in water by flame AAS after preconcentration by solid-phase extraction with Amberlite XAD-2000. Chem Reagents 8:016
Devaraj M, Saravanan R, Deivasigamani RK, Gupta VK, Gracia F, Jayadevan S (2016) Fabrication of novel shape Cu and Cu/Cu2O nanoparticles modified electrode for the determination of dopamine and paracetamol. J Mol Liq 221:930–941
Dou JL, Fu SZ, Wei ZB, Liu JQ, Yin BL, Wei XL, Wu MZ (2010) Dual aqueous phase system formed by ionic liquid and surfactant and its performance in extraction operations. China Surfactant Deterg Cosmetics 4:004
Garg UK, Kaur MP, Garg VK, Sud D (2007) Removal of hexavalent Cr from aqueous solutions by agricultural waste biomass. J Hazard Mater 140:60–68
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removel of some heavey metal ions. Waste Manage 17(8):517–522
Gupta VK, Agarwal S, Saleh TA (2011a) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185(1):17–23
Gupta VK, Jain R, Nayak A, Agarwal S, Shrivastava M (2011b) Removal of the hazardous dye—tartrazine by photodegradation on titanium dioxide surface. Mater Sci Eng: C 31:1062–1067
Gupta VK, Ali I, Saleh TA, Nayaka A, Agarwal S (2012a) Chemical treatment technologies for waste-water recycling-an overview. RSC Adv 2(16):380–6388
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012a) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32(1):12–17
Gupta VK, Mittal A, Jhare D, Mittal J (2012b) Batch and bulk removal of hazardous colouring agent Rose Bengal by adsorption techniques using bottom ash as adsorbent. RSC Adv 2(22):8381–8389
Gupta V K, Kumar R, Nayak A, Saleh TA & Barakat MA (2013a) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review, Adv. Colloid Interf. Sci, 193–194: 24–34
Gupta VK, Yola ML, Eren T, Kartal F, Çağlayan MO, Atar N (2013b) Catalytic activity of Fe@Ag nanoparticle involved calcium alginate beads for the reduction of nitrophenols. J Mol Liq 181:133–141
Gupta VK, Nayak A, Agarwal S (2015) Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ Eng Res 20(1):001–018
Han J, Wang Y, Kang W, Li C, Yan Y, Pan J, Xie X (2010) Phase equilibrium and macrolide antibiotics partitioning in real water samples using a two-phase system composed of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and an aqueous solution of an inorganic salt. Microchim Acta 169(1–2):15–22
International Agency for Research on Cancer (IARC) (1987) Overall evaluations of carcinogenicity: an updating of IARC Monographs. IARC Monogr Eval Carcinog Risks Hum Suppl 1–42(Suppl 7):1–440
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep Sci Technol 38(2):463–481
Jiang B, Feng Z, Liu C, Xu Y, Li D, Ji G (2015) Extraction and purification of wheat-esterase using aqueous two-phase systems of ionic liquid and salt. J Food Sci Tech 52(5):2878–2885
Karthikeyan S, Gupta VK, Boopathy R, Titus A, Sekaran G (2012) A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J Mol Liquids 173:153–163
Khani H, Rofouei MK, Arab P, Gupta VK, Vafaei Z (2010) Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: application to potentiometric monitoring of mercury ion(II). J Hazard Mater 183(1–3):402–409
Kumaraguru N, Santhakumar K, Arunachalam S, Arumugham MN (2006) Synthesis, characterization and micellization behaviour of some surface active mixed-ligand complexes of cobalt (III). Polyhedron 25(17):3253–3260
Lei S, Zhang J, Huang J (2007) Promotion of the surface activity and aggregation ability of sodium dodecylsulfate in aqueous solution by ionic liquid 1-butyl-3-methyl-imidazolium tetrafluoroborate. Acta Phys -Chim Sin 23(11):1657
Li S, He C, Liu H, Li K, Liu F (2005) Ionic liquid-based aqueous two-phase system, a sample pretreatment procedure prior to high-performance liquid chromatography of opium alkaloids. J Chromatogr B 826(1):58–62
Liu SP, Zong ZM, Wei Q, Wei XY (2010) Study on organic compounds in aqueous two phase system phase forming and distribution. Chem Ind Times 24:21–24
Mehta SK, Ram G (2010) Behavior of papain in mixed micelles of anionic–cationic surfactants having similar tails and dissimilar head groups. J Colloid Interf Sci 344(1):105–111
Menéndez-Alonso E, Hill SJ, Foulkes ME, Crighton JS (1999) Speciation and preconcentration of Cr III and Cr VI in waters by retention on ion exchange media and determination by EDXRF. J Anal Atom Spectrom 14(2):187–192
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009a) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interf Sci 337(2):345–354
Mittal A, Mittal J, Malviya A, Gupta VK (2009b) Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J Colloid Interf Sci 340(1):16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010a) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interf Sci 344(2):497–507
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010b) Decoloration treatment of a hazardous triarylmethane dye, light green SF (yellowish) by waste material adsorbents, J. Colloid Interf Sci 342(2):518–527
Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S (2011) Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J colloid interf Sci 362(2):457–462
Rajendran S, Khan MM, Gracia F, Qin JQ, Gupta VK, Arumainathan S (2016) Ce 3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2nanocomposite. Sci Reports 6:31641–31650
Saleh TA, Gupta VK (2011a) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J Colloid Interf Sci 362(2):337–344
Saleh TA, Gupta VK (2012a) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interf Sci 371(1):101–106
Saleh TA, Gupta VK (2012b) Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ Sci Pollu Res 19(4):1224–1228
Saravanan R, Gupta VK, Prakash T, Narayanan V, Stephen A (2013a) Synthesis, characterization and photocatalytic activity of novel Hg doped ZnO nanorods prepared by thermal decomposition method. J Mol Liq 178:88–93
Saravanan R, Joicy S, Gupta VK, Narayanan V, Stephen A (2013b) Visible light induced degradation of methylene blue using CeO 2/V2 O5 and CeO2/CuO catalysts. Mater Sci Eng: C 33(8):4725–4731
Saravanan R, Karthikeyan N, Gupta VK, Sekaran G, Narayanan V, Stephen A (2013c) Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater Sci Eng C 33:91–98
Saravanan R, Karthikeyan N, Gupta VK, Thirumal E, Thangadurai P, Narayanan V, Stephen A (2013d) ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater Sci Eng C 33:2235–2244
Saravanan R, Thirumal E, Gupta VK, Narayanan V, Stephen A (2013e) The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures. J Mol Liq 177:394–401
Saravanan R, Gupta VK, Narayanan V, Stephen A (2014a) Visible light degradation of textile effluent using novel catalyst ZnO/g-Mn2O3. J Taiwan Institute Chem Eng 45:1910–1917
Saravanan R, Gupta VK, Mosquera E, Gracia F (2014b) Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J Mol Liq 198:409–412
Saravanan R, Gracia F, Khan MM, Poornima V, Gupta VK, Narayanan V, Stephen A (2015a) ZnO/CdO nanocomposites for textile effluent degradation and electrochemical detection. J Mol Liq 209:374–380
Saravanan R, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015b) Visible light induced degradation of methyl orange using b-Ag0.333V2O5 nanorod catalysts by facile thermal decomposition method. J Saudi Chem Soc 19(5):521–527
Saravanan R, Khan MM, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015c) ZnO/Ag/Mn2O3 nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and ascorbic acid sensing and antimicrobial activities. RSC Adv 5:34645–34651
Saravanan R, Khan MM, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015d) ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents. J Colloid Interf Sci 452:126–133
Saravanan R, Sacari E, Gracia F, Khan MM, Mosquera E, Gupta VK (2016) Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J Mol Liq 221:1029–1033
Sarubbo LA, Oliveira LA, Porto ALF, Duarte HS, Carneiro-Leão AMA, Lima-Filho JL, Tambourgi EB (2000) New aqueous two-phase system based on cashew-nut tree gum and poly (ethylene glycol). J Chromatogr B 743(1):79–84
Sha O, Zhu X, Feng Y, Ma W (2015) Aqueous two-phase based on ionic liquid liquid–liquid microextraction for simultaneous determination of five synthetic food colourants in different food samples by high-performance liquid chromatography. Food Chem 174:380–386
Smirnova NA, Vanin AA, Safonova EA, Pukinsky IB, Anufrikov YA, Makarov AL (2009) Self-assembly in aqueous solutions of imidazolium ionic liquids and their mixtures with an anionic surfactant. J Colloid Interf Sci 336(2):793–802
Soylak M, Unsal YE, Yilmaz E, Tuzen M (2011) Determination of rhodamine b in soft drink, waste water and lipstick samples after solid phase extraction. Food Chem Toxicol 49(8):1796–1799
Su Z, Zhai H, Chen Z, Zhou Q, Li J, Liu Z (2014) Molecularly imprinted solid-phase extraction monolithic capillary column for selective extraction and sensitive determination of safranine T in wolfberry. Anal Bioanal Chem 406(5):1551–1556
van Berlo M, Luyben KCA, van der Wielen LA (1998) Poly (ethylene glycol)–salt aqueous two-phase systems with easily recyclable volatile salts. J Chromatogr B 711(1):61–68
Wang F, Chen T, Shang Y, Liu H (2011) Two-phase aqueous systems of cetyltrimethylammonium bromide/sodium dodecyl sulfate with and without polyethylene glycol. Korean J Chem Eng 28(3):923–926
Wang W, Zhu X, Yan C (2013) Determination of safranine T in food samples by CTAB sensitised fluorescence quenching method of the derivatives of calix [4] arene. Food Chem 141(3):2207–2212
Zheng XY (2009) Determination of safranine t in foods by ultra performance liquid chromatography-tandem mass spectrometry. J Fuzhou Univ 37(5):752–755
Zhu XS, Jiang RR (2011) Determination of iron(III) by room temperature ionic liquids/surfactant sensitized fluorescence quenching method. J Fluoresc 21(1):385–391
Zhu XS, Zhu XH, Wang B (2006) Cloud point extraction for speciation analysis of inorganic tin in water samples by graphite furnace atomic absorption spectrometry. J Anal Atom Spectrom 21(1):69–73
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (21375117) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Natural Science Foundation of China (21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of Interest
Prof. Xiashi Zhu has a financial relationship with the organizations that sponsored the research: She has received research grants from the National Natural Foundation of China (21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. Songqing Chen, PhD student, declares that he has no conflict of interest. Hao Fu, Master’s student, declares that he has no conflict of interest.
Human and Animal Rights
The research proposed in this article does not contain any studies with human or animals subjects. There are no ethical issues with human or animal subjects in our studies.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Zhu, X., Chen, S. & Fu, H. Separation/Analysis Safranine T in Food Samples Using Surfactant/Ionic Liquid Aqueous Two-Phase Systems. Food Anal. Methods 10, 2764–2771 (2017). https://doi.org/10.1007/s12161-017-0844-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0844-z