Abstract
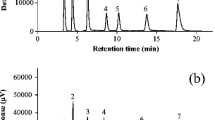
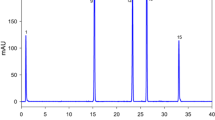
A method based on high-performance liquid chromatography (HPLC) with fluorescence (FL) detection for the simultaneous analysis of phytosterols (stigmasterol, β-sitosterol, campesterol, ergosterol, and fucosterol) and cholesterol was developed. To fluoresceinate the sterols, they were derivatized by 1-anthroyl cyanide to the hydroxyl group at carbon 3 of each sterol skeleton. This HPLC-FL method consists of a C-30 column, an isocratic solution using acetone/acetonitril/hexane/water (71:20:4:5, v/v) as the mobile phase at 1.0 mL min−1 and fluorescence detection at an excitation of 370 nm and an emission of 470 nm. The separation of five phytosterols, cholesterol, and 1-hexacosanol as an internal standard was achieved with sufficient reproducibility and quantitative ability. Our method could evaluate the sterols of land plants such as wood ear fungus, soybean, and parsley, as well as marine algae such as Hiziki (Phaeophyta), Ogonori (Rhodophyta), and Heraiwazuta (Chlorophyta). As a result of the analysis of land plants, wood ear contained a large amount of ergosterol as a precursor of vitamin D2. Soybean contained a large amount of stigmasterol, campesterol, and β-sitosterol. Parsley contained small amounts of these sterols compared with wood ear and soybean. Among the marine algae, Hiziki, Ogonori, and Heraiwazuta contained large amounts of fucosterol, cholesterol, and β-sitosterol, respectively. The compositions of marine algae differed from those of land plants.




Similar content being viewed by others
References
Abdul QA, Choi RJ, Jung HA, Choi JS (2015) Health benefit of fucosterol from marine algae: a review. J Sci Food Agr 96:1856–1866
Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P (2013) Valuable compounds in macroalgae extracts. Food Chem 138:1819–1828
Banlangsawan N, Sanoamuang N (2016) Effect of UV-B irradiation on contents of ergosterol, vitamin D2, vitamin B1 and vitamin B2 in Thai edible mushrooms. Chiang Mai J Sci 43:45–53
Chen Y, Chen J, Jia L (2009) Study of triacontyl-functionalized monolithic silica capillary column for reversed-phase capillary liquid chromatography. J Chromatogr A 1216:2597–2600
Choi JS, Han YR, Byeon JS, Choung SY, Sohn HS, Jung HA (2015) Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J Pharm Pharmacol 67:1170–1178
Escurriol V, Cofán M, Serra M, Bulló M, Basora J, Salas-Salvadó J, Corella D, Zazpe I, Martínez-González MA, Ruiz-Gutiérrez V, Estruch R, Ros E (2009) Serum sterol responses to increasing plant sterol intake from natural foods in the Mediterranean diet. Eur J Nutr 48:373–382
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Furlong ST, Samia JA, Rose RM, Fishman JA (1994) Phytosterols are present in Pneumocystis carinii. Antimicrob Agents Ch 38:2534–2540
Goto J (1983) New sensitive derivatization of hydroperoxide for high-performance liquid chromatography with fluorescence detection. Anal Chim Acta 147:397–400
Govindan M, Hodge JD, Brown KA, Nuñez-Smith M (1993) Distribution of cholesterol in Caribbean marine algae. Steroids 58:178–180
Hoshino M, Watanabe Y, Hamasaki Y, Masubuchi M, Okahira A (1993) Determination of new H2-receptor antagonist Z-300 in plasma by high-performance liquid chromatography with fluorescence detection. Yakugaku Zasshi 113:586–590
Ida K, Hashimoto K, Kamiya M, Muto S, Nakamura Y, Kato K, Mizota M (1996) Stereoselective action of (R*, R*)-(±)-methyl-4-[2-[2-hydroxy-2-(3-chlorophenyl)-ethyl- amino]propyl]-phenoxyacetic acid (BRL37344) on β-adrenoceptors and metabolic chiral inversion. Biochem Pharmacol 52:1521–1527
Ikekawa N, Morisaki N, Tsuda K, Yoshida T (1968) Sterol compositions in some green algae and brown algae. Steroids 12:41–48
Indyk HE (1990) Simultaneous liquid chromatographic determination of cholesterol, phytosterols and tocopherols in foods. Analyst 115:1525–1530
Ito N, Hakamata H, Kusu F (2010) Simultaneous determination of β-sitosterol, campesterol, stigmasterol, and brassicasterol in serum by high-performance liquid chromatography with electrochemical detection. Anal Methods 2:174–179
Jabeur H, Zribi A, Makni J, Rebai A, Abdelhedi R, Bouaziz M (2014) Detection of chemlali extra-virgin olive oil adulteration mixed with soybean oil, corn oil, and sunflower oil by using GC and HPLC. J Agr Food Chem 62:4893–4904
Kubo I (1986) Micro analysis of prostaglandin and ecdysteroids in insects by high-performance liquid chromatography and fluorescence labeling. J Chromatogr 362:61–70
Lagarda MJ, García-Llatas G, Farré R (2006) Analysis of phytosterols in foods. J Pharmaceut Biomed 41:1486–1496
Lin Y-T, Wu S-S, Wu H-L (2007) Highly sensitive analysis of cholesterol and sitosterol in foods and human biosamples by liquid chromatography with fluorescence detection. J Chromatogr A 1156:280–287
Malcolm L (2000) Plant sterol and stanol margarines and health. Brit Med J 320:861–864
Matsushita T, Inoue SI, Tanaka R (2010) An assay method for determining the total lipid content of fish meat using a 2-thiobarbituric acid reaction. J Am Oil Chem Soc 87:963–972
Norcia LN, Rosenthal BE (1966) Sterol content of some plant oils; further observations on fast-reacting sterols. Journal of the American Oil Chemists Society 43(3):168–170
Oka Y, Kiriyama S, Yoshida A (1973) Sterol composition of fruits, fungi, marine algae and tea, coffee and cocoa. Eiyo To Shokuryo 26:317–327
Sánchez-Machado DI, López-Hernández J, Paseiro-Losada P, López-Cervantes J (2004) An HPLC method for the quantification of sterols in edible seaweeds. Biomed Chromatogr 18:183–190
Santos SAO, Vilela C, Freire CSR, Abreu MH, Rocha SM, Silvestre AJD (2015) Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem 183:122–128
Shamsa F, Amjki H (2000) Preparation of 1-anthroyl nitrile as a strong fluorophore for pre-column labeling of hydroxysteroids. Daru 8:37–40
Shimada K, Nakagi T (1996) Studies on neurosteroids. IV. Quantitative determination of pregnenolone in rat brains using high-performance liquid chromatography. J Liq Chromatogr R T 19:2593–2602
Tanaka R, Higo Y, Shibata T, Suzuki N, Hatate H, Nagayama K, Nakamura T (2002) Accumulation of hydroxy lipids in live fish infected with fish diseases. Aquaculture 211:341–351
Vilegas W, Vilegas JHY, Dachtler M, Glaser T, Albert K (2000) Application of on-line C30 RP-HPLC-NMR for the analysis of flavonoids from leaf extract of Maytenus Aquifolium. Phytochem Analysis 11:317–321
Wanaka K, Murui T (1992) Determination of sterols in edible oils and fats by high performance liquid chromatography with fluorescence labeling. J Jpn Oil Chem Soc 41:306–311
Yankah VV (2006) Phytosterols and human health. In: Akoh CC (ed) Handbook of functional lipids. Taylor & Francis, Boca Raton, pp 403–414
Acknowledgements
This study was supported by a grant from the University of Miyazaki, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Meiko Ito declares that she/he has no conflict of interest. Mami Ishimaru declares that she/he has no conflict of interest. Toshiyuki Shibata declares that she/he has no conflict of interest. Hideo Hatate declares that she/he has no conflict of interest. Ryusuke Tanaka declares that she/he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Informed consent was not applicable.
Rights and permissions
About this article
Cite this article
Ito, M., Ishimaru, M., Shibata, T. et al. High-Performance Liquid Chromatography with Fluorescence Detection for Simultaneous Analysis of Phytosterols (Stigmasterol, β-Sitosterol, Campesterol, Ergosterol, and Fucosterol) and Cholesterol in Plant Foods. Food Anal. Methods 10, 2692–2699 (2017). https://doi.org/10.1007/s12161-017-0841-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0841-2