Abstract
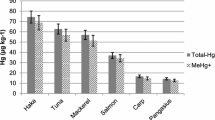
The validation of a method to identify and quantify methylmercury and ethylmercury by liquid-liquid extraction with hexane and determination by using gas chromatography coupled to mass spectrometry was evaluated. The method was validated according to EU Commission Decision 2002/657/EC. Certified reference materials NIST 2976 (mussel tissue) and NMIJ 7402-a (cod fish tissue) and an “in house” full matrix were used. Fractional factorial design was applied, whereas six experimental factors at two levels were employed. Analyses were carried out by two analysts and the intra-days and inter-days significance were considered, thus demonstrating that this method is adequate for the purpose in hand. The decision limits (CCα) and detection capability (CCβ) of the method were 13.88 and 14.55 μg L−1 for methylmercury and 15.74 and 16.07 μg L−1 for ethylmercury, respectively. Accuracy and precision were evaluated by means of recovery experiments at three concentration levels (20, 30, and 40 μg L−1), obtaining recoveries between 70 and 120 % and a coefficient of variation below 10 %. The validated method was successfully applied for determination of methylmercury and ethylmercury in canned tuna, fresh fish, and seafood samples obtained from local markets in Medellin, Colombia.



Similar content being viewed by others
References
Abrankó L, Kmellár B, Fodor P (2007) Comparison of extraction procedures for methylmercury determination by a SPME-GC-AFS system. Microchem Jour 85(1):122–126. doi:10.1016/j.microc.2006.04.001
Alimentarius C (2013). Documento debate sobre la revisión del nivel de referencia para el metilmercurio en el pescado y peces predadores. Moscu: Retrieved from ftp.fao.org/codex/meetings/cccf/cccf7/cf07_16s.pdf
Antignac JP, Le Bizec B, Monteau F, Andre F (2003) Validation of analytical methods based on mass spectrometric detection according to the “2002/657/EC” European decision: guideline and application. Anal Chim Acta 483(1–2):325–334. doi:10.1016/S0003-2670(02)01379-X
Berzas Nevado JJ, Martin-Doimeadios R, Krupp EM, Guzmán Bernardo FJ, Rodríguez Fariñas N, Jiménez Moreno M, Wallace D, Patiño Ropero MJ (2011) Comparison of gas chromatographic hyphenated techniques for mercury speciation analysis. J Chrom A 1218(28):4545–4551. doi:10.1016/j.chroma.2011.05.036
Carrasco L, Díez S, Bayona JM (2007) Methylmercury determination in biota by solid-phase microextraction: matrix effect evaluation. J Chrom A 1174(1–2):2–6. doi:10.1016/j.chroma.2007.09.051
Carrasco L, Díez S, Bayona JM (2009) Simultaneous determination of methyl- and ethyl-mercury by solid-phase microextraction followed by gas chromatography atomic fluorescence detection. J Chrom A 1216(51):8828–8834. doi:10.1016/j.chroma.2009.10.043
Carrasco L, Vassileva E (2014) Determination of methylmercury in marine biota samples: method validation. Talanta 122:106–114. doi:10.1016/j.talanta.2014.01.027
Clémens S, Monperrus M, Donard OFX, Amouroux D, Guérin T (2012) Mercury speciation in seafood using isotope dilution analysis: a review. Talanta 89:12–20. doi:10.1016/j.talanta.2011.12.064
Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. (2002/657/EC) (2002)
Chen SS, Chou SS, Hwang DF (2004) Determination of methylmercury in fish using focused microwave digestion following by Cu2+ addition, sodium tetrapropylborate derivatization, n-heptane extraction, and gas chromatography–mass spectrometry. J Chrom A 1024(1–2):209–215. doi:10.1016/j.chroma.2003.10.015
Chung SW, Chan B (2011) A reliable method to determine methylmercury and ethylmercury simultaneously in foods by gas chromatography with inductively coupled plasma mass spectrometry after enzymatic and acid digestion. J Chrom A 1218(9):1260–1265. doi:10.1016/j.chroma.2010.12.112
Eurachem (2012) Guide Quantifying Uncertainty in Analytical Measurement. Ellison SLR, Williams A
Fan Z, Liu X (2008) Determination of methylmercury and phenylmercury in water samples by liquid–liquid–liquid microextraction coupled with capillary electrophoresis. J Chrom A 1180(1–2):187–192. doi:10.1016/j.chroma.2007.12.010
Hintelmann H, Nguyen H (2005) Extraction of methylmercury from tissue and plant samples by acid leaching. Analytical and Bioanalytical Chemistry 381(2):360–365. doi:10.1007/s00216-004-2878-5
Human Health Risk Assessment of Mercury in Fish and Health Benefits of Fish Consumption (2008) Retrieved from Health Canada website: www.hc-sc.gc.ca/fn-an/pubs/mercur/merc_fish_poisson-eng.php
Li Y, Hu B (2007) Sequential cloud point extraction for the speciation of mercury in seafood by inductively coupled plasma optical emission spectrometry. Spectroc Acta Part B: Atom Spectro 62(10):1153–1160. doi:10.1016/j.sab.2007.07.005
Mason RP, B. J. (2003) Organomercury compounds in the environment organometallic compounds in the environment. John Wiley & Sons, Ltd, pp 57–99
Ong K, L. L., Ghanthimathi S, Ibrahim N, Nasir Z (2014) Speciation: determination of methylmercury in fish samples with HPLC-ICP-MS. In T. I. T. Aris AZ, Harun R, Abdullah AM, Ishak MY (Ed.), From Sources to Solution. Springer Singapore, pp 375–378
Poperechna N, Heumann K (2005) Simultaneous multi-species determination of trimethyllead, monomethylmercury and three butyltin compounds by species-specific isotope dilution GC–ICP–MS in biological samples. Analytical and Bioanalytical Chemistry 383(2):153–159. doi:10.1007/s00216-005-0036-3
Reyes LH, Mizanur Rahman G, Fahrenholz T, Skip Kingston HM (2008) Comparison of methods with respect to efficiencies, recoveries, and quantitation of mercury species interconversions in food demonstrated using tuna fish. Analytical and Bioanalytical Chemistry 390(8):2123–2132. doi:10.1007/s00216-008-1966-3
Reyes LH, Rahman G, Kingston HMS (2009) Robust microwave-assisted extraction protocol for determination of total mercury and methylmercury in fish tissues. Anal Chim Acta 631(2):121–128. doi:10.1016/j.aca.2008.10.044
Rodil R, Carro A, Lorenzo RA, Abuín M, Cela R (2002) Methylmercury determination in biological samples by derivatization, solid-phase microextraction and gas chromatography with microwave-induced plasma atomic emission spectrometry. J Chrom A 963(1–2):313–323. doi:10.1016/S0021-9673(02)00644-1
Sannac S, Fisicaro P, Labarraque G, Pannier F, Potin-Gautier M (2009) Development of a reference measurement procedure for the determination of methylmercury in fish products. Accred Qual Assur 14(5):263–267. doi:10.1007/s00769-009-0509-8
Serafimovski I, Karadjova I, Stafilov T, Cvetković J (2008) Determination of inorganic and methylmercury in fish by cold vapor atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry. Microchem Jour 89(1):42–47. doi:10.1016/j.microc.2007.11.003
Ubillús F, Barberá R, Farré R, Lagarda MJ, Alegría A (2000) Methylmercury and inorganic mercury determination in fish by cold vapour generation atomic absorption spectrometry. Food Chem 71(4):529–533. doi:10.1016/S0308-8146(00)00154-0
Yang L, Mester Z, Sturgeon RE (2003) Determination of methylmercury in fish tissues by isotope dilution SPME-GC-ICP-MS. J Anal Atom Spectrom 18(5):431–436. doi:10.1039/B301299A
Yin XB, W. P., Li Y, Yan XP (2012) 3.22 - Mercury speciation and binding to biomacromolecules. In P. J (ed) Comprehensive sampling and sample preparation. Academic Press, Oxford, pp 435–460
Acknowledgments
The authors express their gratitude to the Departamento Administrativo de Ciencia, Tecnología e Innovación de Colombia, and COLCIENCIAS for funding this research work, and to the Instituto de Ciencia y Tecnología Alimentaria INTAL for the collaboration received.
Conflict of Interest
Claudio Jiménez-Cartagena declares that he has no conflict of interest. Daniel E. León-Pérez declares that he has no conflict of interest. Ana M. Muñoz-Jiménez declares that she has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
León-Pérez, D.E., Muñoz-Jiménez, A.M. & Jiménez-Cartagena, C. Determination of Mercury Species in Fish and Seafood by Gas Chromatography-Mass Spectrometry: Validation Study. Food Anal. Methods 8, 2383–2391 (2015). https://doi.org/10.1007/s12161-015-0120-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0120-z