Abstract
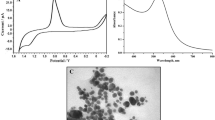
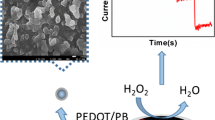
In the present work, an electrochemical biosensor is constructed based on a Nafion nano composite polymer, toluidine blue (TB) and catalase enzyme-modified gold electrode (AuE). The TB molecules were strongly adsorbed on the Nafion/AuE. The electrochemical properties of this biosensor were examined. Cyclic voltammetry was used at various scan rates to investigate the redox properties of the Nafion and TB-modified AuE (Nafion/TB/AuE). The electron transfer coefficient, α, and the electron transfer rate constant, k s, were found to be 0.48 and 12.1 ± 0.3 s−1 in pH 7.0, respectively. The Nafion, TB, and catalase-modified AuE (Nafion/TB/catalase/AuE) exhibited excellent electrocatalytic response to the reduction of hydrogen peroxide (H2O2). Using cyclic voltammetry, kinetic parameters such as electron transfer coefficient, α, and heterogeneous rate constant, k’, were determined for the reduction of H2O2 at this biosensor surface. Differential pulse voltammetry exhibited two linear dynamic ranges and a detection limit of 0.25 μM for H2O2.






Similar content being viewed by others
References
Andrieux CP, Saveant JM (1978) Heterogeneous (chemically modified electrodes, polymer electrodes) vs. homogeneous catalysis of electrochemical reactions. J Electroanal Chem 93:163–168
Bai Y-H, Du Y, Xu J-J, Chen H-Y (2007) Choline biosensors based on a bi-electrocatalytic property of MnO2 nanoparticles modified electrodes to H2O2. Electrochem Commun 9:2611–2616
Bai X, Chen G, Shiu K-K (2013) Electrochemical biosensor based on reduced graphene oxide modified electrode with Prussian blue and poly(toluidine blue O) coating. Electrochim Acta 89:454–460
Bard AJ, Faulkner LR (2000) Electrochemical methods: fundamentals and applications. Wiley, New York
Chandra S, Lokesh KS, Nicolai A, Lang H (2009) Dendrimer-rhodium nanoparticle modified glassy carbon electrode for amperometric detection of hydrogen peroxide. Anal Chim Acta 632:63–68
Chen Q-Y, Li D-H, Yang H-H, Zhu Q-Z, Xu J-G, Zhao Y (1999) Interaction of a novel red-region fluorescent probe, Nile Blue, with DNA and its application to nucleic acids assay. Analyst 124:901–906
Chen S-M, Chuang G-H, Vasantha VS (2006) (Preparation and electrocatalytic properties of the TBO/nafion chemically-modified electrodes. J Electroanal Chem 588:235–243
Gaitan M, Goncales VR, Soler-Illia GJAA, Baraldo LM, de Torresi SIC (2010) Structure effects of self-assembled Prussian blue confined in highly organized mesoporous TiO2 on the electrocatalytic properties towards H2O2 detection. Biosens Bioelectron 26:890–893
Genfa Z, Dasgupta PK, Edgemond WS, Marx JN (1991) Determination of hydrogen peroxide by photoinduced fluorogenic reactions. Anal Chim Acta 243:207–216
Hamidi H, Shams E, Yadollahi B, Esfahani FK (2009) Fabrication of carbon paste electrode containing [PFeW11O39]4− polyoxoanion supported on modified amorphous silica gel and its electrocatalytic activity for H2O2 reduction. Electrochim Acta 54:3495–3500
Hsu CL, Chang KS, Kuo JC (2008) Determination of hydrogen peroxide residues in aseptically packaged beverages using an amperometric sensor based on a palladium electrode. Food Control 19:223–230
Hurdis EC, Romeyn H (1954) Accuracy of determination of hydrogen peroxide by cerate oxidimetry. Anal Chem 26:320–325
Jiang X, Zhu L, Yang D, Mao X, Wu Y (2009) Amperometric ethanol biosensor based on integration of alcohol dehydrogenase with meldola’s blue/ordered mesoporous carbon electrode. Electroanalysis 21:1617–1623
Kok GL, Holler TP, Lopez MB, Nachtrieb HA, Yuan M (1978) Chemiluminescent method for determination of hydrogen peroxide in the ambient atmosphere. Environ Sci Technol 12:1072–1076
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Lazrus AL, Kok GL, Gitlin SN, Lind JA, McLaren SE (1985) Automated fluorimetric method for hydrogen peroxide in atmospheric precipitation. Anal Chem 57:917–922
Li YF, Huang CZ, Li M (2002) Study of the interaction of Azur B with DNA and the determination of DNA based on resonance light scattering measurements. Anal Chim Acta 452:285–294
Lin Y, Cui X, Li L (2005) Low-potential amperometric determination of hydrogen peroxide with a carbon paste electrode modified with nanostructured cryptomelane-type manganese oxides. Electrochem Commun 7:166–172
Lo P-H, Kumar SA, Chen S-M (2008) Amperometric determination of H2O2 at nano-TiO2/DNA/thionin nanocomposite modified electrode. Colloids Surf B 66:266–273
Lobnik A, Cajlakovic M (2001) Sol–gel based optical sensor for continuous determination of dissolved hydrogen peroxide. Sensors Actuator B 74:194–199
Lu Q, Dong X, Li L-J, Hu X (2010) Direct electrochemistry-based hydrogen peroxide biosensor formed from single-layer graphene nanoplatelet–enzyme composite film. Talanta 82:1344–1348
Meng L, Wu P, Chen G, Cai C (2008) Low overpotential detection of NADH and ethanol based on thionine single-walled carbon nanotube composite. J Electrochem Soc 155:F231–F236
Nasirizadeh N, Zare HR, Fakhari AR, Ahmar H, Ahmadzadeh MR, Naeimi A (2011) A study of the electrochemical behavior of an oxadiazole derivative electrodeposited on multi-wall carbon nanotube-modified electrode and its application as a hydrazine sensor. J Solid State Electrochem 15:2683–2693
Nasirizadeh N, Shekari Z, Zare HR, Ardakani SAY, Ahmar H (2013a) Developing a sensor for the simultaneous determination of dopamine, acetaminophen and tryptophan in pharmaceutical samples using a multi-walled carbon nanotube and oxadiazole modified glassy carbon electrode. J Braz Chem Soc 24:1846–1856
Nasirizadeh N, Shekari Z, Zare HR, Shishehbore MR, Fakhari AR (2013b) Electrosynthesis of an imidazole derivative and its application as a bifunctional electrocatalyst for simultaneous determination of ascorbic acid, adrenaline, acetaminophen, and tryptophan at a multi-wall carbon nanotubes modified electrode surface. Biosens Bioelectron 41:608–614
Ping J, Wu J, Fan K, Ying Y (2011) An amperometric sensor based on Prussian blue and poly(o-phenylenediamine) modified glassy carbon electrode for the determination of hydrogen peroxide in beverages. Food Chem 126:2005–2009
Pournaghi-Azar MH, Ahour F, Pournaghi-Azar F (2010) Simple and rapid amperometric monitoring of hydrogen peroxide in salivary samples of dentistry patients exploiting its electro-reduction on the modified/palladized aluminum electrode as an improved electrocatalyst. Sensors Actuator B 145:334–339
Ricci F, Amine A, Moscone D, Palleschi G (2007) A probe for NADH and H2O2 amperometric detection at low applied potential for oxidase and dehydrogenase based biosensor applications. Biosens Bioelectron 22:854–862
Rocha FRP, Ródenas-Torralba E, Reis BF, Morales-Rubio Á, Mdl G (2005) A portable and low cost equipment for flow injection chemiluminescence measurements. Talanta 67:673–677
Skoog DA, Holler FJ, Crouch SR (2007) Principles of instrumental analysis. Thomson Brooks/Cole, London
Song Y, Wang L, Ren C, Zhu G, Li Z (2006) A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized in DNA films on a gold electrode. Sensors Actuator B 114:1001–1006
Song MJ, Hwang SW, Whang D (2010) Amperometric hydrogen peroxide biosensor based on a modified gold electrode with silver nanowires. J Appl Electrochem 40:2099–2105
Thenmozhi K, Narayanan SS (2007) Electrochemical sensor for H2O2 based on thionin immobilized 3-aminopropyltrimethoxy silane derived sol-gel thin film electrode. Sensors Actuator B 125:195–201
Thenmozhi K, Sriman Narayanan S (2007) Amperometric hydrogen peroxide sensor based on a sol-gel-derived ceramic carbon composite electrode with toluidine blue covalently immobilized using 3-aminopropyltrimethoxysilane. Anal Bioanal Chem 387:1075–1082
Tseng K-S, Chen L-C, Ho K-C (2005) Amperometric detection of hydrogen peroxide at a Prussian blue-modified FTO electrode. Sensors Actuator B 108:738–745
Wang W, Wang F, Yao Y, Hu S, Shiu K-K (2010) Amperometric bienzyme glucose biosensor based on carbon nanotube modified electrode with electropolymerized poly(toluidine blue O) film. Electrochim Acta 55:7055–7060
Zare HR, Shekari Z, Nasirizadeh N, Jafari AA (2012) Fabrication, electrochemical characteristics and electrocatalytic activity of 4-((2-hydroxyphenylimino)methyl)benzene-1,2-diol electrodeposited on a carbon nanotube modified glassy carbon electrode as a hydrazine sensor. Catal Sci Technol 2:2492–2501
Zhai X, Li Y, Liu G, Cao Y, Gao H, Yue C, Sheng N (2013) Electropolymerized toluidine blue O functionalized ordered mesoporous carbon-ionic liquid gel-modified electrode and its low-potential detection of NADH. Sensors Actuator B 178:169–175
Zhang K, Zhang N, Xu J, Wang H, Wang C, Shi H, Liu C (2011) Silver nanoparticles/poly(2-(N-morpholine) ethane sulfonic acid) modified electrode for electrocatalytic sensing of hydrogen peroxide. J Appl Electrochem 41:1419–1423
Zheng Y, Lin X-Q (2008) Modified electrode based on immobilizing horseradish peroxidase on nano-gold with choline covalently modified glassy carbon electrode as a base. Chin J Anal Chem 36:604–608
Conflict of Interest
Navid Nasirizadeh has no conflict of interest. Saeedeh hajihosseini has no conflict of interest. Zahra Shekari has no conflict of interest. Masoud Ghaani has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasirizadeh, N., hajihosseini, S., Shekari, Z. et al. A Novel Electrochemical Biosensor Based on a Modified Gold Electrode for Hydrogen Peroxide Determination in Different Beverage Samples. Food Anal. Methods 8, 1546–1555 (2015). https://doi.org/10.1007/s12161-014-0041-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0041-2