Abstract
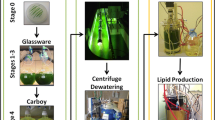
Economically feasible and sustainable energy production from microalgae requires optimization of algal growth, maximization of lipid content, and enhancement of biomass conversion into energy. An innovative, mixed trophic state process with high productivity was implemented to generate microalgae with high lipid content for generating biodiesel and biogas. Auxenochlorella protothecoides, a unicellular green alga, was grown phototrophically to 0.28 dry weight per L (gdw/L) then concentrated to 36 gdw/L for use as an inoculum for a subsequent heterotrophic cultivation to a final density of nearly 120 gdw/L. Simultaneous nitrogen deprivation and glucose supplementation during the heterotrophic stage increased the total lipid content from 16 to 57 % while the triacylglycerol (TAG) fraction of total lipids advanced from 2 to 79 %. Productivity peaked at 4.9 g of biomass/L-h and 1.7 g TAGs/L-h. The extracted lipids, including high levels of oleic, linoleic, and palmitic acids, were converted into biodiesel with a predicted cetane number of 56.4 and low concentrations of long-chain saturated and polyunsaturated fatty acid methyl esters. Both intact microalgal biomass and lipid-extracted algal residues (LEA) were good substrates for anaerobic digestion (AD) with methane yields of 0.6 and 0.4 L/g volatile solids (VS), respectively. These yields represented nearly 80 % of theoretical methane potential. LEA, with a favorable carbon to nitrogen ratio (C:N) of approximately 19:1, is an appropriate substrate for anaerobic microorganisms, most likely because it contains essential nutrients required for microbial digestion. The biochemical composition of the biomass, especially its lipid content, is the major contributor for energy output. As a result, coupling biodiesel production with AD of LEA to generate methane can increase the overall process’ energy output up to 40 %.





Similar content being viewed by others
References
Gladue RM, Maxey JE (1994) Microalgal feeds for aquaculture. J Appl Phycol 6(2):131–141. doi:10.1007/bf02186067
Doucha J, Lívanský K (2011) Production of high-density Chlorella culture grown in fermenters. J Appl Phycol 24(1):35–43. doi:10.1007/s10811-010-9643-2
Bumbak F, Cook S, Zachleder V, Hauser S, Kovar K (2011) Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl Microbiol Biot 91(1):31–46. doi:10.1007/s00253-011-3311-6
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126(4):499–507. doi:10.1016/j.jbiotec.2006.05.002
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresource Technol 97(6):841–846. doi:10.1016/j.biortech.2005.04.008
Wan M-X, Wang R-M, Xia J-L, Rosenberg JN, Nie Z-Y, Kobayashi N, Oyler GA, Betenbaugh MJ (2012) Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol Bioeng 109(8):1958–1964. doi:10.1002/bit.24477
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27(4):409–416. doi:10.1016/j.biotechadv.2009.03.001
Bohutskyi P, Bouwer E (2013) Biogas production from algae and cyanobacteria through anaerobic digestion: a review, analysis, and research needs. In: Lee JW (ed) Advanced Biofuels and Bioproducts. Springer, New York, pp 873–975. doi:10.1007/978-1-4614-3348-4_36
Huss VAR, Frank C, Hartmann EC, Hirmer M, Kloboucek A, Seidel BM, Wenzeler P, Kessler E (1999) Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta). J Phycol 35(3):587–598. doi:10.1046/j.1529-8817.1999.3530587.x
Owen WF, Stuckey DC, Healy JB, Young LY, McCarty PL (1979) Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res 13(6):485–492. doi:10.1016/0043-1354(79)90043-5
Buswell AM, Mueller HF (1952) Mechanism of methane fermentation. Ind Eng Chem 44(3):550–552. doi:10.1021/ie50507a033
Eaton AD, Franson MAH (2005) Standard methods for the examination of water & wastewater, 21st edn. APHA, AWWA, and WEF, New York
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. doi:10.1021/ac60111a017
Maynard LA, Loosli JK (1969) Animal nutrition, 6th edn. McGraw- Hill, New York
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresource Technol 100(1):261–268. doi:10.1016/j.biortech.2008.06.039
Sirisansaneeyakul S, Singhasuwan S, Choorit W, Phoopat N, Garcia JL, Chisti Y (2011) Photoautotrophic production of lipids by some Chlorella strains. Mar Biotechnol 13(5):928–941. doi:10.1007/s10126-010-9355-2
Adams C, Godfrey V, Wahlen B, Seefeldt L, Bugbee B (2013) Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresource Technol 131:188–194. doi:10.1016/j.biortech.2012.12.143
Přibyl P, Cepák V, Zachleder V (2014) Oil overproduction by means of microalgae. In: Bajpai R, Prokop A, Zappi M (eds) Algal Biorefineries. Springer, New York, pp 241–273. doi:10.1007/978-94-007-7494-0_10
Bohutskyi P, Liu K, Kessler BA, Kula T, Hong Y, Bouwer EJ, Betenbaugh MJ, Allnutt T (2014) Mineral and non-carbon nutrient utilization and recovery during sequential phototrophic-heterotrophic growth of lipid-rich algae. Appl Microbiol Biot. doi:10.1007/s00253-014-5655-1
Xiong W, Li X, Xiang J, Wu Q (2007) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biot 78(1):29–36. doi:10.1007/s00253-007-1285-1
Harrison P, Thompson P, Calderwood G (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J Appl Phycol 2(1):45–56. doi:10.1007/bf02179768
Kilham S, Kreeger D, Goulden C, Lynn S (1997) Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshw Biol 38(3):591–596. doi:10.1046/j.1365-2427.1997.00231.x
La Roche J, Geider RJ, Graziano LM, Murray H, Lewis K (1993) Induction of specific proteins in eukaryotic algae grown under iron-, phosphorus-, or nitrogen-deficient conditions. J Phycol 29(6):767–777. doi:10.1111/j.0022-3646.1993.00767.x
Larson TR, Rees TAV (1996) Changes in cell composition and lipid metabolism mediated by sodium and nitrogen availability in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 32(3):388–393. doi:10.1111/j.0022-3646.1996.00388.x
Rhee G (1978) Effects of N:P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol Oceanogr 23(1):10–25
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the green alga Nannochloropsis sp. QII under different nitrogen regimes. J Phycol 23:289–296. doi:10.1111/j.1529-8817.1987.tb04137.x
Tornabene TG, Holzer G, Lien S, Burris N (1983) Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microb Tech 5(6):435–440. doi:10.1016/0141-0229(83)90026-1
Mizuno Y, Sato A, Watanabe K, Hirata A, Takeshita T, Ota S, Sato N, Zachleder V, Tsuzuki M, Kawano S (2013) Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresource Technol 129:150–155. doi:10.1016/j.biortech.2012.11.030
Chen M, Tang H, Ma H, Holland TC, Ng KYS, Salley SO (2010) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresource Technol 102:1649–1655. doi:10.1016/j.biortech.2010.09.062
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21(5):493–507. doi:10.1007/s10811-008-9392-7
Shihira-Ishikawa I, Hase E (1964) Nutritional control of cell pigmentation in Chlorella protothecoides with special reference to the degeneration of chloroplast induced by glucose. Plant cell physiol 5(2):227–240
Shigeji A, Mitsuo M, Eiji H (1965) De- and re-generation of chloroplasts in the cells of Chlorella protothecoides: V. Degeneration of chloroplasts induced by different carbon sources, and effects of some antimetabolites upon the process induced by glucose. Plant cell physiol 6(3):487–498
Hortensteiner S, Chinner J, Matile P, Thomas H, Donnison IS (2000) Chlorophyll breakdown in Chlorella protothecoides: characterization of degreening and cloning of degreening-related genes. Plant Mol Biol 42(3):439–450. doi:10.1023/a:1006380125438
Engel N, Jenny TA, Mooser V, Gossauer A (1991) Chlorophyll catabolism in Chlorella protothecoides. Isolation and structure elucidation of a red bilin derivative. FEBS Lett 293(1–2):131–133. doi:10.1016/0014-5793(91)81168-8
Oshio Y, Hase E (1969) Studies on red pigments excreted by cells of Chlorella protothecoides during the process of bleaching induced by glucose or acetate I. Chemical properties of the red pigments. Plant cell physiol 10(1):41–49
Bohutskyi P, Su C, Liu K, Nasr LK, Byers N, Betenbaugh MJ, Bouwer EJ (2013) Mixotrophic/Heterotrophic algae growth on a low-cost substrate: linking organic waste processing and microalgae cultivation. Poster presentation. In: 7th annual Algae Biomass Summit, Orlando, FL
Li C, Yang H, Xia X, Li Y, Chen L, Zhang M, Zhang L, Wang W (2013) High efficient treatment of citric acid effluent by Chlorella vulgaris and potential biomass utilization. Bioresource Technol 127:248–255. doi:10.1016/j.biortech.2012.08.074
Chen Y-H, Walker TH (2011) Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol Lett 33(10):1973–1983. doi:10.1007/s10529-011-0672-y
O’Grady J, Morgan JA (2010) Heterotrophic growth and lipid production of Chlorella protothecoides on glycerol. Bioproc Biosyst Eng 34(1):121–125. doi:10.1007/s00449-010-0474-y
Pleissner D, Lam WC, Sun Z, Lin CSK (2013) Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresource Technol 137:139–146. doi:10.1016/j.biortech.2013.03.088
Wei A, Zhang X, Wei D, Chen G, Wu Q, Yang S-T (2009) Effects of cassava starch hydrolysate on cell growth and lipid accumulation of the heterotrophic microalgae Chlorella protothecoides. J Industrial Microbiol Biot 36(11):1383–1389. doi:10.1007/s10295-009-0624-x
Li P, Miao X, Li R, Zhong J (2011) In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. J Biomed Biotechnol 2011:1–8. doi:10.1155/2011/141207
Wang W, Zhou W, Liu J, Li Y, Zhang Y (2013) Biodiesel production from hydrolysate of Cyperus esculentus waste by Chlorella vulgaris. Bioresource Technol 136:24–29. doi:10.1016/j.biortech.2013.03.075
Jiang X, Ren C, Hu C, Zhao Z (2013) Isolation and algicidal characterization of Bowmanella denitrificans S088 against Chlorella vulgaris. World J Microb Biot 30(2):621–629. doi:10.1007/s11274-013-1478-y
Lawrence JE (2008) Furtive foes: algal viruses as potential invaders. ICES J Marine Sci 65(5):716–722. doi:10.1093/icesjms/fsn024
Gutman J, Zarka A, Boussiba S (2009) The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur J Phycol 44(4):509–514. doi:10.1080/09670260903161024
Lukavsky J (1970) Phlyctidium scenedesmi, a chytrid destroying an outdoor mass culture of Scenedesmus obliqus. Nova Hedwig 19:775–777
Day JG, Thomas NJ, Achilles-Day UEM, Leakey RJG (2012) Early detection of protozoan grazers in algal biofuel cultures. Bioresource Technol 114:715–719. doi:10.1016/j.biortech.2012.03.015
Zhang Y, Su H, Zhong Y, Zhang C, Shen Z, Sang W, Yan G, Zhou X (2012) The effect of bacterial contamination on the heterotrophic cultivation of Chlorella pyrenoidosa in wastewater from the production of soybean products. Water Res 46(17):5509–5516. doi:10.1016/j.watres.2012.07.025
Bhadury P, Wright P (2004) Exploitation of marine algae: biogenic compounds for potential antifouling applications. Planta 219(4):561–578. doi:10.1007/s00425-004-1307-5
Hellio C, De La Broise D, Dufossé L, Le Gal Y, Bourgougnon N (2001) Inhibition of marine bacteria by extracts of macroalgae: potential use for environmentally friendly antifouling paints. Mar Environ Res 52(3):231–247. doi:10.1016/s0141-1136(01)00092-7
Gupta AB, Shrivastava GC (1965) On antibiotic properties of some fresh water algae. Hydrobiologia 25(1–2):285–288. doi:10.1007/bf00189868
Bacellar Mendes L, Vermelho A (2013) Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol Biofuel 6(1):152. doi:10.1186/1754-6834-6-152
Wolfe GV (2000) The chemical defense ecology of marine unicellular plankton: constraints, mechanisms, and impacts. Biol Bull 198(2):225–244
Natrah FMI, Kenmegne MM, Wiyoto W, Sorgeloos P, Bossier P, Defoirdt T (2011) Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 317(1–4):53–57. doi:10.1016/j.aquaculture.2011.04.038
Defoirdt T, Boon N, Bossier P, Verstraete W (2004) Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture 240(1–4):69–88. doi:10.1016/j.aquaculture.2004.06.031
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86(10):1059–1070. doi:10.1016/j.fuproc.2004.11.002
Knothe G (2007) Some aspects of biodiesel oxidative stability. Fuel Process Technol 88(7):669–677. doi:10.1016/j.fuproc.2007.01.005
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresource Technol 102(1):106–110. doi:10.1016/j.biortech.2010.06.017
Islam M, Magnusson M, Brown R, Ayoko G, Nabi M, Heimann K (2013) Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 6(11):5676–5702. doi:10.3390/en6115676
Tang H, Abunasser N, Garcia MED, Chen M, Simon Ng KY, Salley SO (2011) Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl Energ 88(10):3324–3330. doi:10.1016/j.apenergy.2010.09.013
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48(6):1146–1151. doi:10.1016/j.cep.2009.03.006
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinumby direct transesterification of algal biomass. Energ fuel 23(10):5179–5183. doi:10.1021/ef900704h
Chen Y-H, Huang B-Y, Chiang T-H, Tang T-C (2012) Fuel properties of microalgae (Chlorella protothecoides) oil biodiesel and its blends with petroleum diesel. Fuel 94:270–273. doi:10.1016/j.fuel.2011.11.031
Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98(4):764–771. doi:10.1002/bit.21489
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16(1):143–169. doi:10.1016/j.rser.2011.07.143
Ellis RJ (1979) The most abundant protein in the world. Trends Biochem Sci 4(11):241–244. doi:10.1016/0968-0004(79)90212-3
Manichaikul A, Ghamsari L, Hom EF, Lin C, Murray RR, Chang RL, Balaji S, Hao T, Shen Y, Chavali AK, Thiele I, Yang X, Fan C, Mello E, Hill DE, Vidal M, Salehi-Ashtiani K, Papin JA (2009) Metabolic network analysis integrated with transcript verification for sequenced genomes. Nat Methods 6(8):589–592. doi:10.1038/nmeth.1348
Milner HW (1976) The chemical composition of algae. In: Burlew JS (ed) Algae culture: from laboratory to pilot plant. Carnegie Institution of Washington Publication, Washington, D.C., pp 285–303
Mendez L, Mahdy A, Timmers RA, Ballesteros M, González-Fernández C (2013) Enhancing methane production of Chlorella vulgaris via thermochemical pretreatments. Bioresour Technol 149:136–141. doi:10.1016/j.biortech.2013.08.136
Ras M, Lardon L, Bruno S, Bernet N, Steyer J-P (2011) Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour Technol 102(1):200–206. doi:10.1016/j.biortech.2010.06.146
Eder B, Heinz S (2006) Biogas Praxis: Grundlagen, Planung, Anlagenbau, Beispiele, Wirtschaftlichkeit. Ökobuch Verlag u, Versand, Staufen, Deutschland
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064. doi:10.1016/j.biortech.2007.01.057
Khanal SK (2008) Anaerobic biotechnology for bioenergy production: principles and applications. Wiley-Blackwell, Ames, Iowa
Kroeker EJ, Schulte DD, Sparling AB, Lapp HM (1979) Anaerobic treatment process stability. J WPCF 51(4):718–727. doi:10.2307/25039893
Feinberg DA (1984) Fuel options from microalgae with representative chemical compositions. Technical Report. Solar Energy Research Inst, Golden, CO (USA)
Harun R, Davidson M, Doyle M, Gopiraj R, Danquah M, Forde G (2011) Technoeconomic analysis of an integrated microalgae photobioreactor, biodiesel and biogas production facility. Biomass Bioenerg 35(1):741–747. doi:10.1016/j.biombioe.2010.10.007
Lardon L, Hélias A, Sialve B, Steyer J-P, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43(17):6475–6481. doi:10.1021/es900705jz
Knothe G (2014) A comprehensive evaluation of the cetane numbers of fatty acid methyl esters. Fuel 119:6–13. doi:10.1016/j.fuel.2013.11.020
Uziel M (1978) Solar energy fixation and conversion with algal bacterial systems. PhD thesis, University of California, Berkeley
Ehimen EA, Sun ZF, Carrington CG, Birch EJ, Eaton-Rye JJ (2010) Anaerobic digestion of microalgae residues resulting from the biodiesel production process. App Energ 88(10):3454–3463
Acknowledgements
The authors gratefully acknowledge financial support from US DOE CCS Program (Grant no. DE-FE0001888 to Phycal), US NSF CBET Program (Grant no. 1236691 to JHU), and The Bureau of Education and Cultural Affairs of US Department of State though an International Fulbright Science and Technology Award to Pavlo Bohutskyi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bohutskyi, P., Kula, T., Kessler, B.A. et al. Mixed Trophic State Production Process for Microalgal Biomass with High Lipid Content for Generating Biodiesel and Biogas. Bioenerg. Res. 7, 1174–1185 (2014). https://doi.org/10.1007/s12155-014-9453-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-014-9453-5