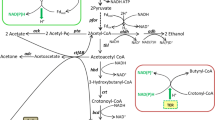
Abstract
Butanol has been considered as a better alternative fuel and it can be produced from anaerobic Clostridial fermentation. Though several enzymes are involved in the biosynthesis of butanol in Clostridia, butanol dehydrogenase (BDH) is understood to play a major role, which catalyzes the conversion of butyraldehyde into butanol at the expenditure of a cofactor NAD(P)H. Recently, the strain Clostridium sp. BOH3 is reported to generate high level of butanol from monosugars. To investigate the BDH activity at various stages of fermentation, BOH3 was cultured in reinforced Clostridial medium with 30 g/l of glucose at 35 °C and the cells were harvested periodically from acid production and solvent production phases. During acid production, NADPH-dependent BDH activity is higher than NADH dependent BDH. Conversely, NADH-BDH activity is predominant during solvent production phase. The optimum pHs for NADH and NADPH-BDH are estimated as pH 6 and 8, respectively. By employing three steps of purification, NADH-BDH is purified to 102-fold with 36 % yield. Subsequent characterization reveals that NADH-BDH is a dimer composed of two subunits depicting the molecular weight of 44 kDa. The peptide finger printing analysis (MS/MS) suggests that the purified protein has higher homology with bifunctional acetaldehyde-CoA and alcohol dehydrogenase of Clostridium acetobutylicum. The extensive kinetic studies show that NADH-BDH follows an ordered sequential bi bi mechanism. The calculated values of K butyraldehyde and K NADH are 8.35 ± 0.25 and 0.076 ± 0.02 mM, respectively, whereas V max is 4.02 ± 0.07 μmol/(mg protein. min). The purified NADH-BDH retains 70 % of its initial activity after 7 days at 4 °C.






Similar content being viewed by others
References
Durre P (2007) Biobutanol: an attractive biofuel. Biotechnol J 2:1525–1534
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energ Combust 37:52–68
Ahn JH, Sang BI, Um Y (2011) Butanol production from thin stillage using Clostridium pasteurianum. Bioresour Technol 102:4934–4937
Kumar M, Gayen K (2011) Developments in biobutanol production: new insights. Appl Energ 88:1999–2012
Radianingtyas H, Wright PC (2003) Alcohol dehydrogenases from thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiol Rev 27:593–616
Li RD, Li YY, Lu LY et al (2011) An improved kinetic model for the acetone–butanol–ethanol pathway of Clostridium acetobutylicum and model-based perturbation analysis. BMC Syst Biol 5 Suppl 1:S12
Zheng YN, Li LZ, Xian M et al (2009) Problems with the microbial production of butanol. J Ind Microbiol Biotechnol 36:1127–1138
Dunlop MJ (2011) Engineering microbes for tolerance to next-generation biofuels. Biotechnol Biofuels 4:32
Dunlop MJ, Dossani ZY, Szmidt HL et al (2011) Engineering microbial biofuel tolerance and export using efflux pumps. Mol Syst Biol 7:487
Huang H, Liu H, Gan YR (2010) Genetic modification of critical enzymes and involved genes in butanol biosynthesis from biomass. Biotechnol Adv 28:651–657
Lehmann D, Lutke-Eversloh T (2011) Switching Clostridium acetobutylicum to an ethanol producer by disruption of the butyrate/butanol fermentative pathway. Metab Eng 13:464–473
Yu M, Zhang Y, Tang IC et al (2011) Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metab Eng 13:373–382
Ezeji T, Blaschek HP (2008) Fermentation of dried distillers' grains and solubles (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour Technol 99:5232–5242
Shi Z, Blaschek HP (2008) Transcriptional analysis of Clostridium beijerinckii NCIMB 8052 and the hyper-butanol-producing mutant BA101 during the shift from acidogenesis to solventogenesis. Appl Environ Microbiol 74:7709–7714
Wang Y, Blaschek HP (2011) Optimization of butanol production from tropical maize stalk juice by fermentation with Clostridium beijerinckii NCIMB 8052. Bioresour Technol 102:9985–9990
Bramono SE, Lam YS, Ong SL et al (2011) A mesophilic Clostridium species that produces butanol from monosaccharides and hydrogen from polysaccharides. Bioresour Technol 102:9558–9563
Gheshlaghi R, Scharer JM, Moo-Young M et al (2009) Metabolic pathways of Clostridia for producing butanol. Biotechnol Adv 27:764–781
Ezeji T, Milne C, Price ND et al (2010) Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl Microbiol Biotechnol 85:1697–1712
Garcíaa V, Päkkiläa J, Ojamob H et al (2011) Challenges in biobutanol production: how to improve the efficiency? Renew Sust Energ Rev 15:964–980
Bennett GN, Rudolph FB (1995) The central metabolic pathway from acetyl-CoA to butyryl-CoA in Clostridium acetobutylicum. FEMS Microbiol Rev 17:241–249
Lee SY, Park JH, Jang SH et al (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101:209–228
Brown SD, Guss AM, Karpinets TV et al (2011) Mutant alcohol dehydrogenase leads to improved ethanol tolerance in Clostridium thermocellum. Proc Natl Acad Sci 108:13752–13757
Green EM, Bennett GN (1996) Inactivation of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. Appl Biochem Biotechnol 57–58:213–221
Adsul MG, Singhvi MS, Gaikaiwari SA et al (2011) Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour Technol 102:4304–4312
Durre P, Kuhn A, Gottwald M et al (1987) Enzymatic investigations on butanol dehydrogenase and butyraldehyde dehydrogenase in extracts of Clostridium acetobutylicum. Appl Microbiol Biotechnol 26:268–272
Petersen DJ, Welch RW, Rudolph FB et al (1991) Molecular cloning of an alcohol (butanol) dehydrogenase gene cluster from Clostridium acetobutylicum ATCC 824. J Bacteriol 173:1831–1834
Terracciano JS, Kashket ER (1986) Intracellular conditions required for initiation of solvent production by Clostridium acetobutylicum. Appl Environ Microbiol 52:86–91
Hiu SF, Zhu CX, Yan RT et al (1987) Butanol–ethanol dehydrogenase and butanol-ethanol-isopropanol dehydrogenase: different alcohol dehydrogenases in two strains of Clostridium beijerinckii (Clostridium butylicum). Appl Environ Microbiol 53:697–703
Welch RW, Rudolph FB, Papoutsakis ET (1989) Purification and characterization of the NADH-dependent butanol dehydrogenase from Clostridium acetobutylicum (ATCC 824). Arch Biochem Biophys 273:309–318
Cleland WW (1963) The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta 67:104–137
Cleland WW (1989) The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. 1963. Biochim Biophys Acta 1000:213–220
Durre P, Fischer RJ, Kuhn A et al (1995) Solventogenic enzymes of Clostridium acetobutylicum: catalytic properties, genetic organization, and transcriptional regulation. FEMS Microbiol Rev 17:251–262
Girbal L, Soucaille P (1998) Regulation of solvent production in Clostridium acetobutylicum. Trends Biotechnol 16:11–16
Chen JS (1995) Alcohol dehydrogenase: multiplicity and relatedness in the solvent-producing Clostridia. FEMS Microbiol Rev 17:263–273
Walter KA, Bennett GN, Papoutsakis ET (1992) Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol 174:7149–7158
Youngleson JS, Jones WA, Jones DT et al (1989) Molecular analysis and nucleotide sequence of the adh1 gene encoding an NADPH-dependent butanol dehydrogenase in the Gram-positive anaerobe Clostridium acetobutylicum. Gene 78:355–364
Ismaiel AA, Zhu CX, Colby GD et al (1993) Purification and characterization of a primary–secondary alcohol dehydrogenase from two strains of Clostridium beijerinckii. J Bacteriol 175:5097–5105
Adolph HW, Zwart P, Meijers R et al (2000) Structural basis for substrate specificity differences of horse liver alcohol dehydrogenase isozymes. Biochemistry 39:12885–12897
Herdendorf TJ, Plapp BV (2011) Origins of the high catalytic activity of human alcohol dehydrogenase 4 studied with horse liver A317C alcohol dehydrogenase. Chem Biol Interact 191:42–47
Rudnick DA, McWherter CA, Rocque WJ et al (1991) Kinetic and structural evidence for a sequential ordered Bi Bi mechanism of catalysis by Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase. J Biol Chem 266:9732–9739
Sekhar VC, Plapp BV (1990) Rate constants for amechanism including intermediates in the interconversion of ternary complexes by horse liver alcohol dehydrogenase. Biochemistry 29:4289–4295
Rudolph FB (1979) Product inhibition and abortive complex formation. Methods Enzymol 63:411–436
Stromberg P, Svensson S, Berst KB et al (2004) Enzymatic mechanism of low-activity mouse alcohol dehydrogenase 2. Biochemistry 43:1323–1328
Acknowledgment
This research was supported by the Competitive Research Programme from Singapore National Research Foundation under Project No.: NRF-CRP5-2009-05. We thank Dr. Ray for providing adhe1 information from genomic data sequence of Clostridium sp. BOH3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajagopalan, G., He, J. & Yang, KL. A Highly Efficient NADH-dependent Butanol Dehydrogenase from High-butanol-producing Clostridium sp. BOH3. Bioenerg. Res. 6, 240–251 (2013). https://doi.org/10.1007/s12155-012-9253-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9253-8